- Home
- Equipment
- usa illinois
- clinical data on brain development
Show results for
Refine by
Clinical Data On Brain Development Equipment Supplied In Usa Illinois
7 equipment items found
Manufactured by:Jointechlabs Inc. based inNorth Barrington, ILLINOIS (USA)
Based on our platform and technology, Jointechlabs is developing therapies for unmet medical needs. Our first therapeutic in clinical development JTL-T-01 is a proprietary injectable stem cell scaffold as a biologic therapy for osteoarthritis, for approval under the FDA’s fast-track program. JTL-T-01 has been designed as an autologous point-of-care therapy enabling the patient’s own ...
Manufactured by:Regenerative Medical Solutions Inc. (RMS) based inMadison, WISCONSIN (USA)
We are developing autologous and allogeneic versions of ILC cellular therapeutic. In the case of autologous ILC cellular therapeutic we will convert the diabetes patients own cells into iPSC cells followed by differentiation of the patient derived iPSC into ILC using our patented and proprietary technology. These patient cell derived ILCs will then be surgically transplanted into the ...
Manufactured by:Regenerative Medical Solutions Inc. (RMS) based inMadison, WISCONSIN (USA)
Regenerative Medical Solutions (RMS) has developed a novel and proprietary technology to transform stem cells into insulin producing cells. Our pancreatic islet like cluster (ILC) cells are morphologically and functionally similar to β islet cells isolated from the human body. They contain glucagon producing alpha cells, somatostatin producing delta cells and insulin producing β islet ...
Manufactured by:Interacoustics based inMiddelfart, DENMARK
A versatile device offering auditory evoked potentials (AEP), auditory steady state response (ASSR), vestibular evoked myogenic potentials (VEMP) and otoacoustic emissions (OAE). Designed to fit seamlessly into your workflow and offers complete reliability and perfect results. ...
Manufactured by:United Metal Fabricators, Inc. (UMF) based inJohnstown, PENNSYLVANIA (USA)
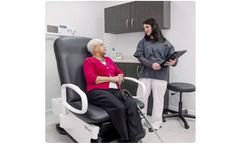
The 4040 Power Back Exam Chair Series are products of extensive research and clinical feedback, developed to improve patient care and streamline the work of healthcare providers. Designed for exams, these chairs meet ADA and U.S. Access Board regulations and accommodates the needs of most ...
Manufactured by:United Metal Fabricators, Inc. (UMF) based inJohnstown, PENNSYLVANIA (USA)
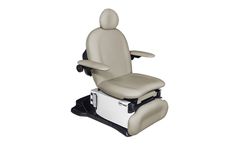
The 4011 Leg-Centric Procedure Chair Series are products of extensive research and clinical feedback, developed to improve patient care and streamline the work of healthcare providers, such as OB/GYN and Oncology professionals. Designed for procedures, these chairs meet ADA and U.S. Access Board regulations and accommodates the needs of most patients. ...
Manufactured by:United Metal Fabricators, Inc. (UMF) based inJohnstown, PENNSYLVANIA (USA)
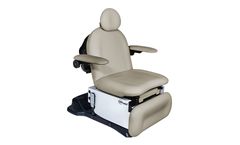
The 4010 Head-Centric Procedure Chair Series are products of extensive research and clinical feedback, developed to improve patient care and streamline the work of healthcare providers, such as ENT, Dermatology, and Plastic Surgery professionals. Designed for head-centric procedures, these chairs meet ADA and U.S. Access Board regulations and accommodates the needs of most ...