- Home
- Equipment
- usa california
- clinical feasibility study
Refine by
Applications
- Actionable Molecular Diagnostic System for Physicians
- Solutions for Glaucoma and Intraocular Pressure (IOP)
- VenoValve - Surgically Implanted Valve for Chronic Venous Disease (CVD)
- VenoValve - Surgically Implanted Valve for Chronic Venous Insufficiency (CVI)
- Potent, Long-Lasting, Infusion Therapy for Patients
- Total Knee Application Solutions for Common Causes of Joint Pain
Clinical Feasibility Study Equipment Supplied In Usa California
109 equipment items found
Manufactured by:Boston Scientific based inMarlborough, MASSACHUSETTS (USA)
FARAFLEX is a large-area focal, deflectable catheter that has been shown to facilitate efficient, point-by-point ablation strategies in clinical studies.3 ...
by:Click Therapeutics, Inc based inNew York, NEW YORK (USA)
Key Features: Controlled Breathing. Social Engagement. Personalized Messaging. Medication Access & Adherence. Digital Diversions. Financial ...
Manufactured by:Boston Scientific based inMarlborough, MASSACHUSETTS (USA)
FARAPOINT is a precision focal ablation catheter designed from the ground up with only PFA in mind. In forthcoming clinical studies, FARAPOINT will aim to facilitate highly targeted therapy while leveraging the safety advantages of FARAPULSE PFA. ...
Manufactured by:Agilent Technologies, Inc. based inSanta Clara, CALIFORNIA (USA)
The SureSelect Target Enrichment System is the most proven target enrichment solution today. This technology enables the capture of genomic targets using long 120nt RNA baits which allow for efficient enrichment of regions of interest facilitating confident variant calling. The SureSelectQXT Reagent Kit is a combined shearing-free transposase-based library prep and target enrichment solution that ...
by:TAE Life Sciences (TLS) based inFoothill Ranch, CALIFORNIA (USA)
TLS’s Alphabeam System includes a compact tandem accelerator-based neutron source and other hardware and software components to provide a complete BNCT solution. Our system is designed to be installed in a hospital setting and can be configured in a single or multi-room BNCT center to meet your clinical, research, and capacity needs. Treatment rooms includes fixed beam, beam shaping ...
Manufactured by:Illumina Inc. based inSan Diego, CALIFORNIA (USA)
The NextSeq 550Dx instrument is FDA regulated and CE-in vitrodiagnostic (IVD) marked, enabling clinical laboratories to develop and perform a wide range of applications, from NGS IVD assays using targeted panels, to clinical research applications that include methods from targeted panels to whole ...
Manufactured by:Varian Medical Systems, Inc. based inPalo Alto, CALIFORNIA (USA)
Next-generation proton therapy with a smaller ...
Manufactured by:Zenflow, Inc. based inSouth San Francisco, CALIFORNIA (USA)
The Spring device gently props open the urethral opening to restore flow. It is elegantly designed using a super-elastic shape-memory material and is custom-fit to provide long-lasting relief. The Spring is delivered through a low-profile flexible scope, providing a more comfortable patient experience. ...
Manufactured by:Bionano based inSan Diego, CALIFORNIA (USA)
Built using proprietary Nanochannel technology, Bionano Chips for the Saphyr and Irys systems linearize DNA, enabling high-speed, high-throughput genome mapping and structural variation detection for a variety of applications including human and clinical ...
Manufactured by:Advanced Brain Monitoring, Inc. based inCarlsbad, CALIFORNIA (USA)
Apnea Guard is a patented non-custom oral appliance that can be fitted in less than 15 minutes by any trained health professional and worn for up to 30 nights. The patented design utilizes a combination of tongue size and sex to select phenotypical features that achieve treatment outcomes superior to the conventional fitting of custom oral ...
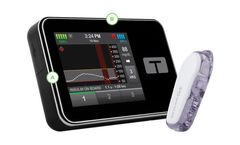
Manufactured by:Tandem Diabetes Care, Inc. based inSan Diego, CALIFORNIA (USA)
When life gets busy, the fear of going low can really get in your way. Our Basal-IQ predictive low-glucose suspend technology can help you spend less time worrying about lows and more time living your ...
Manufactured by:Masimo Corp based inIrvine, CALIFORNIA (USA)
Helps reduce the symptoms of opioid withdrawal. The first FDA-cleared medical device for management of opioid withdrawal symptomsReference. Demonstrated in a clinical study to reduce withdrawal symptoms. May provide relief from opioid withdrawal symptoms, in as soon as 20 ...
Manufactured by:Natus Medical Incorporated (Natus) based inPleasanton, CALIFORNIA (USA)
Natus XactTrode Subdural Electrodes are the most efficient electrode and cable management system on the market. Simplify cable management with one block connector required for every thirty-two (32) contacts. A smarter design means fewer patient cables to connect, providing a more efficient workflow while maximizing the quality of the recordings. This system is designed to integrate seamlessly ...
Manufactured by:PFM Medical, Inc. based inCarlsbad, CALIFORNIA (USA)
The Nit-Occlud PDA occlusion system is the ideal choice for small to medium sized patent ductus arteriosus types. The tightly compact nitinol windings provide safe and effective closure for its patients. This highly flexible and adaptive coil system comes pre-loaded in the delivery system is easily repositionable and retrievable to benefit ...
by:United Rentals Inc. based inStamford, CONNECTICUT (USA)
Pac-Van modular office buildings are truly state-of-the-art in planning and design, in choice and quality of materials, in our demanding construction standards, and in our modern installation techniques. Your temporary or permanent modular office trailer will be constructed off-site as your current site is prepared. This allows you to occupy your modular office building 30-50% sooner. Saving ...
Manufactured by:Magnolia Medical Technologies based inSeattle, WASHINGTON (USA)
Steripath Gen2 is the simple, all-in-one solution clinically proven to reduce false positive blood culture results. Reduce your blood culture contamination rate to improve patient safety and key quality outcomes, while significantly reducing unnecessary antibiotic usage, length of stay and hospital ...
Manufactured by:Credence MedSystems, Inc. based inMenlo Park, CALIFORNIA (USA)
The Credence Connect™ Auto-Sensing Injection System incorporates automatic real-time monitoring of critical injection data into a reusable ergonomic finger grip. The comfortable grip enhances the usability of any syringe while measuring and transmitting injection information in real ...
Manufactured by:Shimadzu Scientific Instruments Inc based inColumbia, MARYLAND (USA)
The MSW2 series offer tools for easily fractionating a predetermined amount of plasma from a small amount of blood, for nonclinical animal experiments. Also, since the entire process, from sampling to fractionation of a predetermined amount of plasma, can be completed with devices only, so human errors are minimized. Use case was introduced at the luncheon seminar of CPSA. CPSA: Clinical & ...
Manufactured by:Emmaus Medical, Inc based inTorrance, CALIFORNIA (USA)
ENDARI [L-glutamine oral powder] is indicated to reduce the acute complications of sickle cell disease in adults and children 5 years and ...
Manufactured by:Advanced Brain Monitoring, Inc. based inCarlsbad, CALIFORNIA (USA)
The Stat X-Series mobile EEG systems offer healthcare professionals cost-effective and practical solutions for on-call and portable EEG in minutes. The headset design enable mobility during recordings, while also minimizing the artifacts and noise typically associated with traditional, wired EEG systems. Standard software and SDK are included to enable the development of applications with ...