- Home
- Equipment
- north america
- clinical send out testing
Show results for
Refine by
Applications
- Actionable Molecular Diagnostic System for Physicians
- Medical cannabis solutions for regulatory pathway sector
- Duodenal Mucosal Resurfacing System (DMR) for Type 2 Diabetes
- Duodenal Mucosal Resurfacing System (DMR) for Clinical Research Studies
- Duodenal Mucosal Resurfacing System (DMR) for T2Di Study
- Duodenal Mucosal Resurfacing System (DMR) for Healthcare Professionals
- Venous Stent System for Clinical Trial
- Medical cannabis solutions for mental health costs sector
- Clean air solutions for allergens area
- Clean air solutions for pollution area
- Clean air solutions for bad odor area
- Clean air solutions for bacteria area
- Clean air solutions for virus area
Clinical Send Out Testing Equipment Supplied In North America
170 equipment items found
Manufactured by:Nuritas Ltd. based inDublin, IRELAND
PeptiForce™ is clinically tested and contains 4 targeted peptides that improve the body’s ability to metabolise sugar, it can address needs across the health ...
Manufactured by:Nuritas Ltd. based inDublin, IRELAND
PeptiYouth™ is clinically tested to improve cellular regeneration with a unique double action which increases cellular matrix reconstruction and reduces ...
Manufactured by:Withings Health Solutions based in, MEXICO
Body is an ultra accurate and easy-to use clinically tested smart scale that offers a complete weight-tracking experience tailored to individuals seeking easy, effective weight ...
Manufactured by:International Flavors & Fragrances (IFF) based inNew York, NEW YORK (USA)
Curcugen™ is a highly concentrated, clinically studied curcumin ingredient, fully derived from a turmeric base with a high, 50% curcuminoid concentration. It is derived from turmeric oleoresin as base ingredient with a more comprehensive profile of turmeric bioactives than the industry-standard starting material, 95% curcuminoids. The oleoresin is the native matrix where turmeric’s ...
Manufactured by:Brooklyn ImmunoTherapeutics (BTX) based inSan Diego, CALIFORNIA (USA)
Mesenchymal stem/stromal cells (MSC) have undergone extensive clinical testing for many diseases and have consistently demonstrated safety. In addition, the immunomodulatory properties of MSC have been well characterized. However, adult-tissue-derived MSC have shown inconsistent therapeutic efficacy, significant variability among samples, and limited proliferative capacity. On the other ...
Manufactured by:Beta Bionics, Inc. based inConcord, MASSACHUSETTS (USA)
At Beta Bionics, tech is our thing. Long story short, we’re a medical device company currently focused on the design, development, and launch of an insulin delivery device - the iLet® Bionic Pancreas. CAUTION: The iLet® Bionic Pancreas is an investigational device limited by Federal (or United States) law to investigational use. Not available for sale. To be clear, the iLet Bionic ...
Manufactured by:United Biologics, Inc. based inIrvine, CALIFORNIA (USA)
The Transsetpal Model is our transparent, compliant model featuring access across the atrial septum, between the left and right atria the fossa opening (16mm wide x _____ tall). This model includes external/internal jugulars down to the iliacs and a modified/enhanced structural heart. The removal of the right ventricle allows for better visualization of the mitral valve and chordae. ...
Manufactured by:QURE Healthcare based inSan Francisco, CALIFORNIA (USA)
A clinical game for primary care providers that tests professional competency of healthcare clinical practices to measure performance and ...
Manufactured by:Sekisui Diagnostics, LLC based inBurlington, MASSACHUSETTS (USA)
Blood Clotting Accelerant 2.5% enhances blood clotting with having very low influence on clinical test results. Accelerates blood clotting, Prevent blood sticking, Easy to coat for the tube, Low influence on clinical test ...
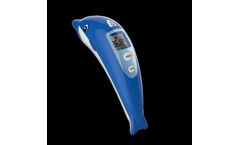
Manufactured by:Microlife Corporation based inWidnau, SWITZERLAND
The new Microlife non-contact thermometer NC 400, further to it's reliability, accuracy, and easy handling, allows gentle and non-invasive measurement of body temperature without touching the patient. The unique dolphin design was specifically developed to make a measurement more pleasant for children. ...
Distributed by:UCP Biosciences Inc. based inSan Jose, CALIFORNIA (USA)
Single panel u-CassettesTM can be custom configured with any CLIA waived, FDA cleared, or FUO drug tests. u-Cassettes may also be customized to include up to 6 types of adulterant tests (see tables below). ...
Distributed by:UCP Biosciences Inc. based inSan Jose, CALIFORNIA (USA)
Multi-Panel u-CassettesTM can be custom configured with any CLIA waived, FDA cleared, or FUO drug tests. u-Cassettes may also be customized to include up to 6 types of adulterant tests (see tables below). ...
Manufactured by:Shiphrah Biomedical Inc. based inToronto, ONTARIO (CANADA)
Introducing the PrenaBelt™, the first and only clinically-tested and published positional therapy device for use during sleep-in-pregnancy with internationally-demonstrated safety and efficacy in minimizing the amount of time spent sleeping on the back during late pregnancy in the home setting without otherwise disturbing sleep quantity or ...
Manufactured by:Eberbach Corporation based inVan Buren Charter Township, MICHIGAN (USA)
Eberbach’s Clinical Rotator E2500 is designed for V.D.R.l., Mazzini, Kline, boerner-Jones-luken, cardiolipin, other serological tests, and clinical applications within a speed range of 30 to 220 rpm. A 13” square platform with sponge rubber pad orbits 5” above the bench. The unit measures 10.5 x 12.4 x 5.2” and is mounted on four suction feet to reduce creeping. At a ...
Manufactured by:United Biologics, Inc. based inIrvine, CALIFORNIA (USA)
The Left & Right Tortuous ICA models include all seven segments of the internal carotid artery, from the cervical (C1) portion to the communicating (C7) segment. The models feature a tapered siphon representing stenoses. The left and right ICAs can be purchased individually or as a ...
Manufactured by:Microlife Corporation based inWidnau, SWITZERLAND
The new Microlife non-contact thermometer NC 400, further to it's reliability, accuracy, and easy handling, allows gentle and non-invasive measurement of body temperature without touching the patient. The unique dolphin design was specifically developed to make a measurement more pleasant for children. ...
Manufactured by:More Diagnostics Inc. based inLos Osos, CALIFORNIA (USA)
More Diagnostics’ Rap/Tac/CsA Control has assayed values for CEDIA® Cyclosporine /QMS® Tacrolimus (For CEDIA/QMS methods - available only from Thermo Fisher Scientific) Tacrolimus and Cyclosporine. ...
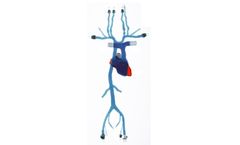
Manufactured by:United Biologics, Inc. based inIrvine, CALIFORNIA (USA)
The Transseptal Model is our transparent, compliant model featuring access across the atrial septum, between the left and right atria. This model includes external/internal jugulars down to the iliacs, and a complete structural heart including the coronary sinus. ...
Manufactured by:Interacoustics based inMiddelfart, DENMARK
Customize your Titan for screening, diagnostic and advanced clinical testing. Match the modules with your current needs – and upgrade when your challenges change. The Titan delivers Impedance, ABRIS, OAE and the revolutionary Wideband Tympanometry. ...
Manufactured by:Diagnosys LLC based inLowell, MASSACHUSETTS (USA)
The Dark Adaptometry module uses the ColorDome™ or ColorFlash™ stimulator to perform a full or partial field dark adaptation to accurately measure retinal parameters such as cone sensitivity, rod sensitivity and the rod-cone break point. The dark adaptometry test may be run for single or dual eye measurements or a combination. Protocols include the Marmor Test as well as a wide array ...