Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
Clinical Specimen Equipment Supplied In Europe
14 equipment items found
Manufactured by:Copan Italia s.p.a. based inBrescia, ITALY
eSwab – Copan’s Liquid Amies Elution Swab – collection and transport system is our multipurpose media intended for the collection and transport of clinical specimens containing aerobes, anaerobes, fastidious bacteria, viruses and ...
Manufactured by:Trina Bioreactives Ag based inNaenikon, SWITZERLAND
Customize your own panel. Up to 5000 Donors in matched anticoagulants. ...
Manufactured by:Trina Bioreactives Ag based inNaenikon, SWITZERLAND
Seroconversion, performance and assay validation panels. ...
Manufactured by:Trina Bioreactives Ag based inNaenikon, SWITZERLAND
Any anticoagulant can be paired and matched ...
Manufactured by:Trina Bioreactives Ag based inNaenikon, SWITZERLAND
Over 50’000 donors for biospecimen from globally different diagnostic and demographic individuals. (Europe, Asia, Americas, Africa) . Different Matrixes (Serum, EDTA, LiHep, etc.) & Multibleeds 1 - 800 ml per Donor. Clinical Data: Patient Data (Age / Gender / GT) Detailed Anamnesis ...
Manufactured by:bioactiva diagnostica GmbH based inBad Homburg, GERMANY
Intended Use:- InTray® SAB-FungID™ contains Sabouraud’s dextrose agar, a nonselective medium used to aid in the detection of dermatophyte fungi Nfrom clinical specimens with mixed microbiota. Expiry date:- 10-12 Months, Certification:- CE IVD, Company:- Biomed Diagnostics, Inc., Storage condition:- 2 - 8° C, Materials Provided:- InTray ...
Manufactured by:bioactiva diagnostica GmbH based inBad Homburg, GERMANY
Intended Use:- InTray® SAB-FungID™ w/ CC contains Sabouraud’s dextrose agar with chloramphenicol and cycloheximide, a selective medium used to aid in the detection of dermatophyte fungi from clinical specimens with mixed microbiota. Expiry date:- 10-12 Months, Certification:- CE IVD, Company:- Biomed Diagnostics, Inc., Storage condition:- 2 - ...
Manufactured by:Copan Italia s.p.a. based inBrescia, ITALY
Copan Universal Transport Medium (UTM®) system is intended for the collection, transport, and preservation of clinical specimens containing Viruses, Chlamydia, Mycoplasma, and ...
Manufactured by:Medical Wire & Equipment based inCorsham,, UNITED KINGDOM
∑–MM™ is the only inactivation media that inactivates deadly pathogens in 60 secs. Has been proven to be effective against SARS-CoV-2. This media can be used for microorganisms, bacteria & viruses, including Coronavirus. The liquid media is great for culture and molecular techniques. It has been proven to be keep the RNA and DNA stable for at least 21 days at room temperature ...
Manufactured by:OraSure Technologies based inBethlehem, PENNSYLVANIA (USA)
The OraQuick® Ebola Rapid Antigen Test has been granted De Novo 510(k) clearance by the U.S. Department of Health and Human Services for the following intended use: The OraQuick® Ebola Rapid Antigen Test is an in vitro diagnostic single-use immunoassay for the qualitative detection of antigens from viruses within the Ebolavirus genus but does not differentiate between these viruses. ...
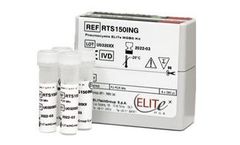
Manufactured by:ELITechGroup based inPuteaux, FRANCE
Pneumocystis pneumonia (PCP) is an opportunistic disease caused by invasion of the unicellular fungus Pneumocystis jirovecii (PJ). In lungs PJ exists in trophic and cystis form and it is one of the most frequent and severe opportunistic infections in people with weakened immune systems. PCP a major cause of morbidity and mortality among HIV-infected patient, nowadays reduced with ART, moreover, ...
Manufactured by:OraSure Technologies based inBethlehem, PENNSYLVANIA (USA)
The OraQuick® Ebola Rapid Antigen Test has been granted De Novo 510(k) clearance by the U.S. Department of Health and Human Services for the following intended use: The OraQuick® Ebola Rapid Antigen Test is an in vitro diagnostic single-use immunoassay for the qualitative detection of antigens from viruses within the Ebolavirus genus but does not differentiate between these viruses. ...
Manufactured by:Lepu Medical Technology (Beijing) Co.,Ltd. based inChangping District, CHINA
SARS-COV2 Antigen Rapid Test Card contains a gold-labeled novel coronavirus N protein monoclonal antibody pre-coated on the bonding pad and a paired novel coronavirus N protein monoclonal antibodies ?xed in the test line (T) and corresponding antibodies in the quality control line (C). SARS-COV2 Antigen Rapid Test Kit can detect the virus from the ?rst phase of infection (2-3 days before ...
Manufactured by:BioMaxima SA based inLublin, POLAND
Rapid chromatographic immunoassay for the qualitative detection of specific antigens to SARS-CoV-2 present in human nasopharynx. Result in: 15-30 minutes. Simple procedure. Specimen: nasopharyngeal swab. Sensitivity and specificity: As recommended by WHO. Test kits contains all needed materials. ...