- Home
- Equipment
- usa massachusetts
- clinical trials
Show results for
Refine by
Applications
- Microbiome Therapeutics for Patients and Physicians
- Flagship Coil for Major Depressive Disorder (MDD) Treatment
- Flagship Coil for Anxious Depression Treatment
- Flagship Coil for Obsessive-Compulsive Disorder (OCD) Treatment
- Flagship Coil for Smoking Addiction Treatment
- Flagship Coil for Bipolar Disorder Treatment
- Flagship Coil for Post Traumatic Stress Disorder Treatment Treatment
- Flagship Coil for Schizophrenia (Negative Symptoms) Treatment
- Flagship Coil for Alzheimer’s Disease Treatment
- Flagship Coil for Autism Disorder Treatment Technology
Clinical Trials Equipment Supplied In Usa Massachusetts
66 equipment items found
by:Curavit Clinical Research based inBoston, MASSACHUSETTS (USA)
The Curavit DCT Platform is the digital manifestation of our decentralized clinical trial services. From operations, through data collection, to patient experience, our proprietary platform manages every aspect of your trial and provides insight into your data. Our platform, reflects our expert processes, is built from industry-leading ...
Manufactured by:BioSensics LLC based inNewton, MASSACHUSETTS (USA)
BioSensics offers a fully customizable sensor-integrated ePRO solution to support event-driven and timely data collection from ...
Manufactured by:BioSensics LLC based inNewton, MASSACHUSETTS (USA)
BioSensics provides devices and digital technologies for collection and analysis of speech data during site visits and at predefined frequencies in the home ...
Manufactured by:BioSensics LLC based inNewton, MASSACHUSETTS (USA)
BioSensics offers wearable sensors for objective and centralized rating of motor symptoms during site visits and for continuous home ...
Manufactured by:BioSensics LLC based inNewton, MASSACHUSETTS (USA)
LEGSys and BalanSens are first FDA-registered wearable devices for objective assessment of gait, balance, fall risk and ...
Manufactured by:BioSensics LLC based inNewton, MASSACHUSETTS (USA)
BioSensics offers wearable sensors and digital technologies for remote monitoring of falls and ...
Manufactured by:BioSensics LLC based inNewton, MASSACHUSETTS (USA)
PAMSys is a clinical-grade wearable sensor for continuous remote monitoring of physical activity, sleep, posture, gait parameters and ...
Manufactured by:Transgene based inIllkirch-Graffenstaden, FRANCE
A particularly innovative immunotherapy, TG4050 is the lead myvac® candidate. This individualized treatment is based on the specific mutations that are identified in each patient’s ...
Manufactured by:Neofect based inWatertown, MASSACHUSETTS (USA)
The Neofect Smart Glove was designed with neuroplasticity in mind. Training programs promote motor learning and brain reorganization to improve arm and hand function for adults rehabilitating from stroke. Sensor technology detects and records movement data. Neofect Smart Glove is a high-tech rehab device that measures movements of the forearm, wrist, and digits with accelerometer and bending ...
Manufactured by:Transgene based inIllkirch-Graffenstaden, FRANCE
BT-001 is the first oncolytic virus from Invir.IO™ and is optimized to act as a Trojan horse. Two specific “weapons” have been integrated into the Vaccinia viral DNA: an anti-CTLA4 antibody, which was developed by BioInvent, and the cytokine GM-CSF, which triggers the body’s immune ...
Manufactured by:Transgene based inIllkirch-Graffenstaden, FRANCE
A new generation of multifunctional oncolytic virus, TG6002 has been designed to combine the mechanism of oncolysis (targeted destruction of the cancer cell) with the production of chemotherapy (5-FU), directly into the ...
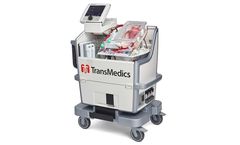
Manufactured by:TransMedics, Inc. based inAndover, MASSACHUSETTS (USA)
The Only Heart Device Currently Under FDA Review for Utilized and Unutilized Donor Organs. The OCS Heart is a portable, warm perfusion, and monitoring system designed to keep a donor heart at a human-like, metabolically active state. By monitoring key parameters of the functioning heart, physicians may use their medical judgement to assess a potentially suitable heart’s condition and ...
by:Aphios Corporation based inWoburn, MASSACHUSETTS (USA)
Despite the widespread use of the 5-HT3 receptor antagonist anti-emetics such as Palonosetron (Aloxi; Eisai), Ondansetron (Zofran®; GSK), Granisetron (Kytril®; Roche Pharmaceuticals), and Dolasetron (Anzemet®; Aventis), CINV continues to be reported by up to 70% of adult patients receiving highly emetogenic chemotherapy agents and 58% of school-age and adolescent-age children ...
by:MC10 based inLexington, MASSACHUSETTS (USA)
BioStamp nPoint is an FDA 510(k) cleared medical device designed to collect medical grade, clinical quality bio-metric, physiological and eCOA data in a clinical trial ...
Manufactured by:AOBiome Therapeutic, LLC based inCambridge, MASSACHUSETTS (USA)
We have completed six Phase 2 clinical programs and multiple pre-clinical programs. Over 1,000 patients have been enrolled in our clinical trials since 2016. All of those patients except for 28 in our open label pediatric study have been enrolled in randomized double-blind placebo controlled clinical ...
by:T2 Biosystems, Inc. based inLexington, MASSACHUSETTS (USA)
Detection of biothreat pathogens in 3-5 hours— direct from whole blood: The T2Biothreat® Panel, which runs on the T2Dx® Instrument, is a direct-from-blood test panel that provides results in 3-5 hours and simultaneously detects six biothreat pathogens identified as threats by the U.S. Government, including B. anthracis, F. tularensis, B. mallei , B. pseudomallei, Y. pestis, and R. ...
by:Iterative Health, Inc. based inCambridge, MASSACHUSETTS (USA)
AI-assisted clinical trial recruitment: the way forward. Our AI Recruitment technology specifically addresses patient recruitment pain points in the clinic. It’s also great for accelerating the approval timeline on novel IBD ...
by:Cartesian Therapeutics based inGaithersburg, MASSACHUSETTS (USA)
Selecta’s first ImmTORTM + gene therapy candidate MMA-101 for methylmalonic acidemia (MMA) is expected to enter clinical trials the end of 2021. MMA is a rare metabolic disease that may lead to metabolic acidosis and hyperammonemia and is associated with long-term complications including feeding problems, developmental delay, intellectual disability, and ...
by:Vor Biopharma based inCambridge, MASSACHUSETTS (USA)
Licensed from the National Institutes of Health, VCAR33 is a CD33-directed chimeric antigen receptor T cell (CAR-T) therapy. A T cell therapy using the same CAR construct as VCAR33 is being studied in a multi-site Phase 1/2 clinical trial as an autologous monotherapy bridge-to-transplant for relapsed and/or refractory AML patients, sponsored by the National ...
by:Vertex Pharmaceuticals based inBoston, MASSACHUSETTS (USA)
Vertex is focused on developing transformative medicines for people with serious and life-threatening diseases. To do this, we conduct clinical trials to assess the safety and effectiveness of investigational medicines, which, if established, will help us obtain approvals from regulatory authorities. These approvals, in turn, are required before medicines can be ...