- Home
- Equipment
- usa california
- clinical validation
Refine by
Clinical Validation Equipment Supplied In Usa California
53 equipment items found
Manufactured by:AliveCor, Inc based inMountain View, CALIFORNIA (USA)
The world’s only 6-lead personal EKG. Clinically validated to give you a more detailed view of your ...
by:Click Therapeutics, Inc based inNew York, NEW YORK (USA)
CT-132 is under development as a clinically-validated digital program for ...
by:Click Therapeutics, Inc based inNew York, NEW YORK (USA)
Clickadian is under development as a clinically-validated fully digital program for ...
by:Click Therapeutics, Inc based inNew York, NEW YORK (USA)
Clickheart is under development as a clinically-validated digital program for acute coronary ...
by:Click Therapeutics, Inc based inNew York, NEW YORK (USA)
CT-161 is under development as a clinically-validated digital program for overactive ...
Manufactured by:CytomX Therapeutics, Inc. based inSouth San Francisco, CALIFORNIA (USA)
Bristol Myers Squibb is enrolling patients in the dose escalation phase of a Phase 1/2a clinical trial (NCT03994601) of a second anti-CTLA-4 Probody, BMS-986288, based on a modified version of Yervoy® (ipilimumab), to evaluate a CTLA-4-directed Probody therapeutic alone or in combination with Opdivo® (nivolumab) in patients with selected advanced solid cancers. CTLA-4, a ...
by:Qardio, Inc. based inSan Francisco, CALIFORNIA (USA)
Track and manage COVID-19 symptoms over time. QardioSpO2 is a clinically validated pulse oximeter that measures the blood oxygen saturation levels (SpO2) and pulse rate of the body via the fingertip sensor. Compatible with iOS and Android devices. ...
Manufactured by:BridgeBio Pharma, Inc. based inPalo Alto, CALIFORNIA (USA)
In PH1, loss of function mutation in the AGXT gene results in accumulation of glyoxylate, which is converted into oxalate and leads to kidney stones and organ damage. Targeting GO is a clinically validated approach to reduce urinary oxalate by lowering the concentration of glyoxylate. The first-in-human Phase 1 trial of BBP-711 for hyperoxaluria was initiated in ...
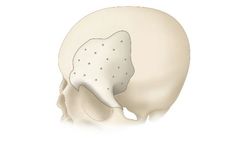
Manufactured by:Bioplate based inPlacentia, CALIFORNIA (USA)
The CRANIOFIT Customized PEEK Plates are tailored to address intricate cranial deformities, offering a solution designed for precision in craniofacial reconstructive surgery. These plates are crafted from polyetheretherketone (PEEK), a clinically validated bioengineered material known for its robustness, comfort, and longevity. They are lightweight and customized ...
by:Qardio, Inc. based inSan Francisco, CALIFORNIA (USA)
Track your complete heart health on your smartphone, Get a clinically validated, electrocardiograph trace for deeper heart health insights, Enjoy your lifestyle with an innovative and easy to wear design, Share data with your doctor, automatically. QardioCore is the world’s first ECG monitor designed to provide continuous medical grade data while fitting ...
Manufactured by:Tulip Medical Products based inSan Diego, CALIFORNIA (USA)
The replenishing capabilities of TRUE NanoFat (TNF) have opened up new opportunities to improve overall health so that patients can get back to living a normal life. Outpatient practitioners can now effectively use their patients' own adipose tissue to help the body heal itself and restore function with little or no downtime. The SoftFoot™ MicroFat + TRUE NanoFat (TNF) Procedure Kit. For ...
Manufactured by:A&D Medical based inSan Jose, CALIFORNIA (USA)
A classic blood pressure monitor with the benefits of speed and accuracy at the touch of a button ...
Manufactured by:Spinal Elements, Inc. based inCarlsbad, CALIFORNIA (USA)
The Posterior ALIF: Minimal access, maximum footprint from a posterior approach. Multi-dimensional interbody options to restore lordosis1,2. Clinically-proven to resist long-term ...
Manufactured by:QURE Healthcare based inSan Francisco, CALIFORNIA (USA)
A clinical game for primary care providers that tests professional competency of healthcare clinical practices to measure performance and ...
Manufactured by:NewGen Surgical based inSan Rafael, CALIFORNIA (USA)
Sustainable Design Needle counter box is: 100% renewable bio-based material. Reduces CO2e by 25% - 50%. Free of intentionally added BPA or BPA derived plastics, mercury, phthalates and PVC. PFAS ...
Manufactured by:A&D Medical based inSan Jose, CALIFORNIA (USA)
Advanced, multi-user blood pressure monitor that provides trusted accuracy and reliable measurements every ...
Manufactured by:A&D Medical based inSan Jose, CALIFORNIA (USA)
A classic wrist blood pressure monitor with the benefits of speed and accuracy at a touch of a ...
Manufactured by:Penumbra, Inc. based inAlameda, CALIFORNIA (USA)
The Penumbra Coil 400™ (PC400™) is a large-volume platinum coil. With its larger .020″ (.508 mm) diameter, PC400 is designed to enable higher packing densities using fewer coils, leading to potential efficiencies in procedure duration, time under fluoroscopy, and treatment ...
Manufactured by:A&D Medical based inSan Jose, CALIFORNIA (USA)
Advanced, multi-user blood pressure monitor that provides trusted accuracy and reliable measurements every ...
Manufactured by:Integrated Endoscopy based inIrvine, CALIFORNIA (USA)
An Advanced, Single-use, Arthroscopic Innovation; Single-use nuvis is a surgical device that addresses the many issues hampering conventional reusable ...