Refine by
Coronary Disease Equipment & Supplies
68 equipment items found
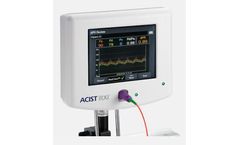
Manufactured by:ACIST Medical Systems based inEden Prairie, MINNESOTA (USA)
Non-hyperemic index for coronary physiology. ACIST diastolic pressure ratio (dPR), using the ACIST RXi Rapid Exchange System and Navvus MicroCatheter, provides a non-hyperemic alternative for physiological assessment of coronary ...
Manufactured by:SOFIE based inDulles, VIRGINIA (USA)
N13-Ammonia is a radioactive diagnostic agent for Positron Emission Tomography (PET) indicated for diagnostic PET imaging of the myocardium under rest or pharmacologic stress conditions to evaluate myocardial perfusion in patients with suspected or existing coronary artery ...
Manufactured by:Bioprojet based inParis, FRANCE
Kengrexal, co-administered with acetylsalicylic acid (ASA), is indicated for the reduction of thrombotic cardiovascular events in adult patients with coronary artery disease having undergone a percutaneous coronary intervention (PCI), who have not received an oral P2Y12 receptor inhibitor prior to the intervention and in whom the oral ...
Manufactured by:Diazyme Laboratories, Inc. based inPoway, CALIFORNIA (USA)
Diazyme's HDL-Cholesterol Assay is a direct dual liquid stable reagent system. Convenient instrument specific packaging options including Roche™ Hitachi 917 series, Beckman AU (400/600/640/680), Beckman Synchron CX, LX and DXC. The assay utilizes Diazyme's proprietary PVSPEGME coupled technology which provides outstanding performance. The assay features a linear range of 1.06 to 184.8 ...
Manufactured by:Circle Cardiovascular Imaging Inc. based inCalgary, ALBERTA (CANADA)
Specialized tools for the assessment of coronary artery disease using cardiac CT. Read and report Cardiac CT with zero-click coronary artery segmentation and quantifying calcium scoring powered by ...
Manufactured by:CeloNova BioSciences, Inc. based inCarlsbad, CALIFORNIA (USA)
Our proprietary COBRA SG drug-coated PTCA balloon includes a bio-active medical coating designed to improve adhesion of organic and inorganic substrates and optimize drug delivery. Developed at a leading, international medical university, the novel coating enables a unique and efficient excipient to carry a non-proliferative drug on the surface of PTCA balloons and has the flexibility to be ...
Manufactured by:Nextern Inc based inWhite Bear Lake, MINNESOTA (USA)
We have expertise in producing microcatheters used in neurovascular, cardiovascular, and peripheral vascular applications. Our goal is to design and manufacture innovative products that provide better patient outcomes and advance the field of medical ...
Manufactured by:Diazyme Laboratories, Inc. based inPoway, CALIFORNIA (USA)
Diazyme's LDL-Cholesterol Assay is a direct dual liquid stable reagent system. Reagent transfer is eliminated for most chemistry systems with a choice of convenient instrument specific packaging options including Roche™ Hitachi 917 series, Beckman AU (400/600/640/680), Beckman Synchron CX, LX and DXC. The assay utilizes Diazyme's proprietary PVSPEGME coupled technology which provides ...
Manufactured by:Concept Medical Inc. based inTampa, FLORIDA (USA)
Novel, First of its kind Sirolimus drug coated balloon catheter for the treatment coronary artery disease. MagicTouch is intended for in-stent restenosis, small vessels, bifurcation lesions and de-novo lesions ...
Manufactured by:ACME Portable Machines, Inc. based inNew Taipei City, TAIWAN
It is a patented algorithm that analyzes 10 second 12 lead ECG captured by either our own QED 2000 or a certified off-the-shelf ECG machine, and it aims to assist in detection of myocardial ischemia related to Coronary Artery Disease ...
Manufactured by:Sinocare based inChangsha, CHINA
The PalmLab® (Model: SLX-120&SLX-121): Lipid and Blood Glucose Monitoring System is for the quantitative measurement of total cholesterol(TC), high density lipoprotein(HDL) cholesterol, triglycerides(TG), and glucose(GLU). A Chol/HDL ratio and estimated values for low density lipoprotein(LDL) cholesterol and non-HDL cholesterol are calculated by the analyzer. ...
by:Supira Medical based inLos Gatos, CALIFORNIA (USA)
Developing a low-profile, high-flow percutaneous ventricular assist device (pVAD) for high risk coronary intervention and cardiogenic ...
Manufactured by:Vitalis, LLC based inNew York, NEW YORK (USA)
ER (extended-release) niacin currently has six FDA approved indications and has been prescribed to millions of patients in the U.S. alone (Jackevicius et al.). However, a well-known side effect of treating with ER niacin is Niacin Flush (Mills et al.). The Niacin Flush is a historical term for the medical syndrome of the “skin on fire” experience of these patients. Niacin Flush can be ...
Manufactured by:Bluesail Medical Co., Ltd based inZibo, CHINA
EXCEL is the first biodegradable polymer sirolimus eluting stent (BP-SES), being widely used in percutaneous coronary intervention (PCI) for patients with coronary atherosclerotic disease. A large number of evidence-based clinical data have demonstrated its safety and efficacy and more than a million patients have benefited since EXCEL launched ...
Manufactured by:Athens Research & Technology Inc. based inAthens, GEORGIA (US) (USA)
Buy Purified Native Human Apolipoprotein AI (ApoA1), Human Plasma. Bulk Qty Available. 75% of Apo A in HDL is Apo AI. Levels of Apo AI are inversely related to the risk of coronary heart disease. Apo AI is also thought to activate LCAT (lecithin cholesterol acyl tranferase). In normal plasma, Apo AI levels range from 90-130 mg per 100 ml. ...
Manufactured by:Elixir Medical Corporation based inMilpitas, CALIFORNIA (USA)
The DynamX Drug Eluting Coronary Bioadaptor System is a significant innovation in the treatment of coronary artery disease. Going beyond drug-eluting stents (DES), DynamX represents one of the most significant breakthroughs in implant design in the past 30 ...
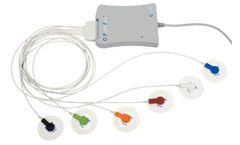
Manufactured by:APC Cardiovascular Ltd. based inHartford, UNITED KINGDOM
Using thoracic electrical bioimpedance, the PhysioFlow™ incorporates unique and patented signal waveform morphology analysis algorithm to measure cardiac output and other hemodynamic parameters, thus overcoming the accuracy problems associated with impedance baseline (Z0) measurements, even during maximal exercise. Its HD-Z™ high-tech filter stabilizes signals even under extreme ...
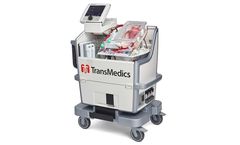
Manufactured by:TransMedics, Inc. based inAndover, MASSACHUSETTS (USA)
The Only Heart Device Currently Under FDA Review for Utilized and Unutilized Donor Organs. The OCS Heart is a portable, warm perfusion, and monitoring system designed to keep a donor heart at a human-like, metabolically active state. By monitoring key parameters of the functioning heart, physicians may use their medical judgement to assess a potentially suitable heart’s condition and ...
Manufactured by:CARDIONOVUM GmbH based inBonn, GERMANY
Unmatched XLIMUS clinical performance. The ultimate DES for complex artery lesions. XLIMUS is considered as the ultimate coronary DES stent system to treat complex coronary artery disease by reaching and crossing the most challenging ...
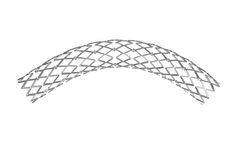
Manufactured by:amg International GmbH based inWinsen, GERMANY
The ARTHOSPico is a multicellular Cobalt-Chromium stent that offers strength, stability, and flexibility for treating the most difficult lesions. The Co-Cr Stent is indicated for patients with coronary heart disease. It is inserted in coronary vessels with a diameter of 2.0 to 4.0 mm. It is used for the treatment of de-novo and restenotic ...