Show results for
Refine by
Covid 19 Equipment Supplied In China
62 equipment items found
Manufactured by:Shanghai Fosun Long March Medical Science Co., Ltd based inBaoshan, CHINA
Features: Easy to use. Affordable. Highly portable, requiring no instrumentation. Reliable, with 95.2% sensitivity and 99.6% specificity. Fast, with results in 15 ...
Manufactured by:NanoEnTek, Inc. based inGuro-gu, SOUTH KOREA
The En-swer™ COVID-19 RT-PCR Kit detects E, N, and RNaseP genes of SARS-CoV-2 qualitatively through real-time reverse-transcription polymerase chain reaction. It can be used for rapid detection and outbreak control of COVID-19. ...
Manufactured by:Genabio Diagnostics Inc. based inBedford, MASSACHUSETTS (USA)
Intended use: iHealth® COVID-19 Antigen Rapid Test is a lateral flow assay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2. This test is authorized for non-prescription home use with self-collected anterior nasal (nares) swab samples from individuals aged 15 years or older with symptoms of ...
Manufactured by:Genabio Diagnostics Inc. based inBedford, MASSACHUSETTS (USA)
Intended Use: INDICAID™ COVID-19 Rapid Antigen Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARSCoV-2 in direct anterior nasal swab specimens from individuals who are suspected of COVID-19 by their healthcare provider within the first five (5) days of symptom ...
Manufactured by:EirGenix, Inc based inNew Taipei City, TAIWAN
It can detect COVID-19 and get result in 10 minute! ...
Manufactured by:DiaSino Laboratories Co., Ltd. based inZhengzhou, CHINA
Nasal swab: Sensitivity: 95.38% Specificity: ...
Manufactured by:Maccura Biotechnology Co., Ltd. based inChengdu, CHINA
Maccura’s SARS-CoV-2 Fluorescent PCR Kit (for the COVID-19 Coronavirus) has been approved by CE & NMPA. And it was used in many hospitals from EU & Asia & Others and got high ...
Manufactured by:HymonBio Co.,Ltd based inSuzhou, CHINA
Hymon COVID-19 antigen rapid test kit is a lateral flow immunoassay for the qualitative detection of SARS-COV-2 antigen (nucleocapsid protein) with nasal swabs or saliva during the acute phase of infection. It only takes 15 minutes to complete the test and the test is also non-invasive. ...
Manufactured by:Genabio Diagnostics Inc. based inBedford, MASSACHUSETTS (USA)
cIntended Use: Fosun COVID-19 RT-PCR Detection Kit is a real-time RT-PCR test intended for the qualitative detection of nucleic acid from the SARS-CoV-2 in upper and lower respiratory specimens (such as anterior nasal swabs, mid-turbinate nasal swabs, nasopharyngeal swabs, oropharyngeal swabs, sputum, lower respiratory tract aspirates, bronchoalveolar lavage, and ...
Manufactured by:Ningbo Greetmed Medical Instruments Co.,Ltd. based inNingbo, CHINA
Rapid testing for SARS-CoV-2 antigen within 15 minutes. Facilitates patient treatment decisions quickly. Simple, time-saving procedure. All necessary reagents provided & no equipment needed. High sensitivity and ...
Manufactured by:NanoEnTek, Inc. based inGuro-gu, SOUTH KOREA
The FREND COVID-19 Neutralization Ab is a point-of-care testing (POCT) which can be used to check whether a patient has developed the immune response to SARS-CoV-2 using human serum or plasma ...
Manufactured by:Wuhan Fine Biotech Co., Ltd. based inWuhan, CHINA
COVID-19 Spike Protein Accquant ELISA Kit allows for the in vitro quantitative determination of COVID-19 Sipke ELISA Kit concentrations in serum, plasma, tissue homogenates and other biological fluids. ...
Manufactured by:Pro-Health Product Ltd based inGuangzhou, CHINA
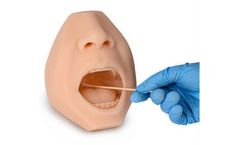
The must-have trainer is ideal for use in visual demonstration and training of a nasal & oropharyngeal swab sample collection not only for the test and diagnosis of COVID-19 but also for many respiratory viruses and airborne ...
Manufactured by:Wuhan HealthCare Biotechnology Co., Ltd. based inWuhan, CHINA
This kit is used for the in vitro qualitative detection of suspected cases of pneumonia caused by New Coronavirus infection, patients with suspected clusters, other patients who need to be diagnosed or differentially diagnosed with the New Coronavirus infection, and nasopharyngeal swabs, oropharyngeal swabs, and bronchoalveolar lavage fluid samples of patients with mutation beads for the New ...
Manufactured by:Xiamen Biotime Biotechnology Co., Ltd. based inXiamen, CHINA
COVID-19 Ag & Flu A/B Combo Self Test is a colloidal gold immunochromatography intended for the in vitro rapid, simultaneous qualitative detection and differentiation of N protein from SARS-CoV-2, influenza A and influenza B directly from nasal swab specimens obtained from individuals, who are suspected of COVID-19, influenza ...
Manufactured by:Liferiver Bio-Tech (United States) Corp. based inLa Jolla, CALIFORNIA (USA)
LightCycler 1.0 (Internal control can't be used for this system); LightCycler 2.0; PE5700, MJ-Opticon & other single color systems; ABI7000, ABI7300, ABI7500, ABI7900, ABI StepOne, StepOne plus, MJ-Opticon2, MJ-chromo4, MX3000P, MX3005P, Smart Cycler II, Rotor-Gene 6000, LightCycler 480, CFX 96, Slan 96, MIC, iCycler iQ4, iCycler iQ5 & other multi-color ...
Manufactured by:Getein Biotechnology Co.,Ltd. based inNanjing, CHINA
Test Item: SARS-CoV-2 Antigen. Sample Type: Human nasal swab. Test Time: 10-15 min. Methodology: Colloidal ...
Manufactured by:Getein Biotechnology Co.,Ltd. based inNanjing, CHINA
Test Item: SARS-CoV-2 Antigen. Sample Type: Human nasal swab. Test Time: 15 min. Methodology: Immunofluorescence ...
Manufactured by:Getein Biotechnology Co.,Ltd. based inNanjing, CHINA
Can be used as a aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection, it should not be used to diagnose acute SARS-CoV-2 infection. ...
Manufactured by:NanoEnTek, Inc. based inGuro-gu, SOUTH KOREA
Quantitative immunoassay analyzing instrument that utilizes disposable cartridge to provide in vitro diagnostics ...