- Home
- Equipment
- north america
- covid 19 igg igm rapid
Show results for
Refine by
Covid 19 Igg Igm Rapid Equipment Supplied In North America
16 equipment items found
Manufactured by:ACON Laboratories, Inc. based inSan Diego, CALIFORNIA (USA)
Detects IgG/IgM antibodies to SARS-CoV-2. Product Features: High Sensitivity and Specificity - Relative Sensitivity: 99.1% (95.12 – 99.98 %)*, Relative Specificity: 98.2% (96.29 – 99.27%)*, Accuracy: 98.5% (96.9 – 99.5%)*, *95% Confidence Intervals. Device Highlights: Test Cassette, Detects IgG and IgM, Small sample volume, Multiple sample formats, Fast results. Specimen ...
Manufactured by:Elabscience based inHouston, TEXAS (USA)
COVID-19 (Corona Virus Disease) is an infectious disease caused by the most recently discovered coronavirus. COVID-19 IgG/IgM Rapid Test ( Serum/Plasma/Whole Blood) is a rapid chromatographic immunoassay for the qualitative of IgG and ...
Manufactured by:Azure Biotech Inc. based inHouston, TEXAS (USA)
This test has not been FDA cleared or approved. This test has been authorized by FDA under an EUA for use by authorized laboratories. It is a point of care test for fingerstick whole blood specimens only. The user should be trained in the procedure. Wear appropriate protective attire for your safety when handling patient ...
Distributed by:Hardy Diagnostics based inSanta Maria, CALIFORNIA (USA)
The RightSign COVID-19 IgG/IgM Rapid Test Cassette is a lateral flow immunochromatographic assay for the detection of SARS-CoV-2 antibodies in venous whole blood, serum, or plasma. The COVID-19 IgG/IgM Rapid Test is intended for ...
Manufactured by:Premier Biotech, Inc. based inMinneapolis, MINNESOTA (USA)
The Premier Biotech COVID-19 IgG/IgM Rapid Test Cassette is a lateral flow immunochromatographic assay for the detection of SARS-CoV-2 antibodies in venous whole blood, serum or plasma. The COVID-19 IgG/IgM Rapid Test Cassette ...
Manufactured by:Able Diagnostics, Inc. based inSan Diego, CALIFORNIA (USA)
The 1st Point-of-Care (POC) Antibody Test for COVID-19 with Fingerstick Whole Blood Authorized by FDA!. Click the link to review FDA NEWS RELEASE on Sept 23, 2020 in details: "FDA Authorizes First Point-of-Care Antibody Test for COVID-19" about Assure COVID-19 ...
Manufactured by:CTK Biotech, Inc. based inPoway, CALIFORNIA (USA)
The Positivia COVID-19 Ag Rapid Test External Control Kit is intended for use with the OnSite COVID-19 Ag Rapid Test (REF ...
Distributed by:Hardy Diagnostics based inSanta Maria, CALIFORNIA (USA)
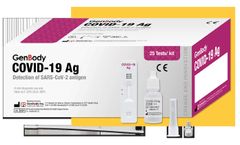
A rapid immunoassay for the simultaneous direct detection and differential diagnosis of SARS-CoV-2, Influenza Type A and Type B antigen from anterior nasal (AN) or nasopharyngeal (NP) swab specimens. Kit includes extraction buffer vials and flocked nasopharyngeal (NP) swabs for superior specimen collection and patient comfort, and provides a visual read-out in 15 minutes with no equipment needed! ...
Manufactured by:Genabio Diagnostics Inc. based inBedford, MASSACHUSETTS (USA)
Intended use: iHealth® COVID-19 Antigen Rapid Test is a lateral flow assay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2. This test is authorized for non-prescription home use with self-collected anterior nasal (nares) swab samples from individuals aged 15 years or older with symptoms of COVID-19 within the first 7 days of symptom onset. This test is ...
Manufactured by:Ellume Limited based inEast Brisbane, AUSTRALIA
In partnership with global diagnostics company QIAGEN®, Ellume has developed two rapid COVID-19 tests: the QIAreach™ SARS-CoV-2 Antigen Test**** for active infections and the QIAreach™ Anti-SARS-CoV-2 Total Test** for past infections. Both tests are now available in the U.S. The tests are run on the eHub platform, an easy-to-use and portable digital device that provides results in ...
Manufactured by:Genabio Diagnostics Inc. based inBedford, MASSACHUSETTS (USA)
Intended Use: INDICAID™ COVID-19 Rapid Antigen Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARSCoV-2 in direct anterior nasal swab specimens from individuals who are suspected of COVID-19 by their healthcare provider within the first five (5) days of symptom onset. Anterior nasal swab specimens may be collected by a ...
Distributed by:Labex Canada Inc. based in, ONTARIO (CANADA)
The COVID-19 Vaccine IgG Rapid Test is a lateral flow immunoassay intended for the qualitative detection of antibodies binding to the Receptor Binding Domain (RBD) of the spike protein of the SARS-CoV-2 virus or COVID-19 vaccine in human serum, plasma or whole blood. The kit is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating ...
Manufactured by:Accel Diagnostics based inHouston, TEXAS (USA)
A Rapid Test for the Quantitation of the Titer of Antibody Against the Spike Protein of the SARS-COV-2 VIRUS. Accel Diagnostics’ rapid quantitative COVID-19 serology assay for the detection and qualification anti-Spike SARS-COV-2 antibody concentration (IgG) is now ...
Distributed by:Hardy Diagnostics based inSanta Maria, CALIFORNIA (USA)
An immunochromatographic rapid diagnostic test (RDT) intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct nasopharyngeal (NP) or anteriornasal (AN) swab specimens. Authorized for use under an Emergency Use Authorization (EUA) and for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate ...
Manufactured by:Benevolent based inLondon, UNITED KINGDOM
The emerging global threat from COVID-19 demanded immediate action. With cases rising rapidly, we set up a specialist team to use our AI-drug discovery platform to search for existing drugs — already proven safe — that could be repurposed to treat the virus. We are the only AI company to successfully identify a drug to treat COVID-19. Together by building bespoke drug repurposing ...
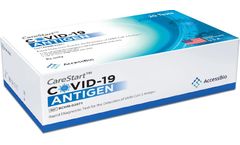
Manufactured by:Access Bio based inSomerset, NEW JERSEY (USA)
The CareStart™ COVID-19 Antigen Test is a lateral flow immunochromatographic assay intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in nasopharyngeal or anterior nasal swab specimens directly collected from individuals who are either suspected of COVID-19 by their healthcare provider within the first five days of symptom onset or from individuals ...