Refine by
Cross Linking Procedure Equipment & Supplies
3 equipment items found
Manufactured by:Glaukos Corporation based inSan Clemente, CALIFORNIA (USA)
iLink is the first and only FDA-approved corneal cross-linking procedure that slows or halts the progression of keratoconus and helps preserve vision. Watch this video for unique patient and doctor perspectives about progressive keratoconus and learn how the condition is treated with iLink™ corneal ...
Manufactured by:Surflay Nanotec GmbH based inBerlin, GERMANY
Air-filled polyvinyl alcohol capsules in sizes ranging from 3-4 μm are produced in Surflaylabs. Thanks to a special cross-linking procedure, these bubbles are stable for months, hydrophilic and uncharged. Due to their low density, they slowly float in water to the ...
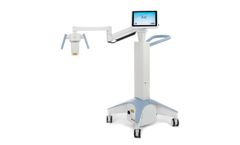
Manufactured by:Glaukos Corporation based inSan Clemente, CALIFORNIA (USA)
The iDetect KC program makes advanced topography more accessible than ever before. For a fraction of the price, optometrists can bring this essential diagnostic tool into their practice so they ...