Refine by
Degenerative Lumbar Equipment & Supplies
12 equipment items found
Manufactured by:SpineVision based inAntony, FRANCE
The FLEX +2 system is an innovative sterile rod designed for degenerative lumbar dynamic stabilization or topping off assembly. The FLEX +2 is a dynamic device to be implantable whether during a percutaneous approach or open surgeries. ...
Manufactured by:Surgical Solutions based inSan Juan, PUERTO RICO
Cervical, thoraco-lumbar, degenerative & deformity products that correct and improve spinal conditions?. ...
Manufactured by:EOS MEDICAL SOLUTIONS (EOSMED) based inSofia, BULGARIA
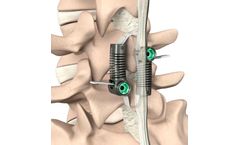
Alecta Lumbar Peek Cage is manufactured from MRI and CT-compatible PEEK material, which does not result in permanent lesions. PLIF is particularly useful for the elimination of neurological compression and for the correction of degenerative lumbar deformities such as spondylolisthesis, scoliosis, or disc space collapse. ...
Manufactured by:EOS MEDICAL SOLUTIONS (EOSMED) based inSofia, BULGARIA
Alecta Lumbar Peek Cage is manufactured from MRI and CT-compatible PEEK material, which does not result in permanent lesions. PLIF is particularly useful for the elimination of neurological compression and for the correction of degenerative lumbar deformities such as spondylolisthesis, scoliosis, or disc space collapse. ...
Manufactured by:SpineVision based inAntony, FRANCE
The Ulis is a powerful & intuitive top loading pedicle screw system specially designed to simplify open surgeries for lumbar degenerative spine. The Ulis ergonomic and compact set allows surgeons to perform a versatile construct with a large choice of rods: Titanium alloy , Cobalt Chrome alloy & Flex +2 dynamic or topping off ...
by:Aurora Spine, Inc. based inToronto, ONTARIO (CANADA)
The ZIP ULTRA implant is designed for stabilization and load sharing in T1-S1 thoracolumbar fusion procedures, specifically for the treatment of degenerative disease, lumbar spinal stenosis, spondylolisthesis, trauma, and/or tumor. The proprietary ZIP ONE-STEP™ locking mechanism eliminates the use of a set screw. Each ZIP ULTRA implant features a large ...
by:Empirical Spine, Inc. based inSan Carlos, CALIFORNIA (USA)
LimiFlex is intended to provide segmental stabilization following decompression for grade 1 lumbar degenerative spondylolisthesis with spinal stenosis. LimiFlex is implanted through a dorsal approach, typically through the same incision used for the surgical ...
by:Aurora Spine, Inc. based inToronto, ONTARIO (CANADA)
The ZIP LP implant is designed for stabilization and load sharing during T1-S1 thoracolumbar fusion procedures, specifically for the treatment of degenerative disc disease, lumbar spinal stenosis, spondylolisthesis, trauma, and/or tumor. The proprietary ZIP ONE-STEP™ locking mechanism eliminates the use of a set screw. Each ZIP LP implant features a large ...
by:Aurora Spine, Inc. based inToronto, ONTARIO (CANADA)
The ZIP 51 implant is designed for stabilization and load sharing during T1 - S1 thoracolumbar fusion procedures, specifically for the treatment of degenerative disc disease, lumbar spinal stenosis, spondylolisthesis, trauma, and/or tumor. The proprietary ZIP ONE-STEP™ locking mechanism eliminates the use of a set screw. Each ZIP 51 implant features a large ...
Manufactured by:3D Medical based inMarolles-en-Brie, FRANCE
In-vivo study of porous structures made in titanium via SLM allows to observe the excellent osteointegration of this type of ...
Manufactured by:Genesis Medical Plastics based inCypress, TEXAS (USA)
As one of the most complicated structures in the human body, the spinal column must be handled carefully, and with an eye for the future. Surgical intervention to correct conditions like degenerative disc disease (DDD), lumbar spinal stenosis, degenerative scoliosis or degenerative spondylolisthesis is a challenge, and the risks ...
Manufactured by:Ankasa Regenerative Therapeutics based inLa Jolla, CALIFORNIA (USA)
Stem cell renewal and maintenance and tissue regeneration are critical for normal health and well-being. Wnt pathway interactions are crucial to maintenance of these processes. Ankasa is the first to produce human Wnt proteins in a manufacturing setting suitable for use in humans. Our first clinical approach will be the demonstration of improved outcomes after spinal fusion surgeries. Our product ...