Refine by
Degenerative Spine Equipment & Supplies
18 equipment items found
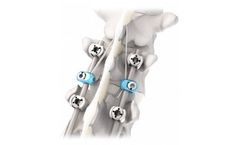
Manufactured by:IMPLANET based inMartillac, FRANCE
Innovative and unique concept in the market addressing degenerative spine surgeons concerns, Jazz™ PF is the Tethering Solution. Soft landing of spinal posterior constructs. Tethering augmentation is an option in mitigating the sudden stiffness change. Several tethering linkage ...
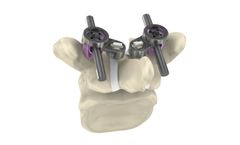
Manufactured by:IMPLANET based inMartillac, FRANCE
Innovative and unique concept in the market addressing degenerative spine surgeons concerns, Jazz™ Cap SP (Screw Protection) is the only current solution that allows direct coupling with a pedicle screw (Jazz™ Screw) to supplement it. Significantly increase its primary stability in situations of poor quality of the patient’s ...
Manufactured by:SpineVision based inAntony, FRANCE
The Ulis is a powerful & intuitive top loading pedicle screw system specially designed to simplify open surgeries for lumbar degenerative spine. The Ulis ergonomic and compact set allows surgeons to perform a versatile construct with a large choice of rods: Titanium alloy , Cobalt Chrome alloy & Flex +2 dynamic or topping off ...
Manufactured by:Hoogland Spine Products GmbH based inFrankfurt am Main, GERMANY
Biportal Endoscopic Spine System, BESS, comes out to be one of new trends of treatment of degenerative spine disorders. Literally it has a deeper origin from bilaterally (bilateral approach, one(left) side for scoping and the other(right side) for working) biportal arthroscopic approach by Dr. ...
Manufactured by:Neuro France Implants based inLa Ville aux Clercs, FRANCE
S.E.S EVOLUTION® system is intended for thoraco-lumbo-sacral posterior ...
Manufactured by:Neo Medical Sa based inVillette, SWITZERLAND
Degenerative scoliosis, describes a side-to-side curvature of the spine caused by degeneration of the facet joints and intervertebral discs which are the moving parts of the spine. This adult degeneration and resulting spinal asymmetry can occur slowly over time as a person ages, and may cause symptoms including lower back and/or leg pain. ...
Manufactured by:Neuro France Implants based inLa Ville aux Clercs, FRANCE
The S.E.S + is a system for scoliosis composed of screws, 3 hooks and rods. Thanks to its connector hook, it allows to strengthen the construct area thanks to 4 ...
Manufactured by:Neuro France Implants based inLa Ville aux Clercs, FRANCE
The PM cages (in titanium or in PEEK OPTIMA®) are intended for the fusion and restoration of the intersomatic space between two or more cervical ...
Manufactured by:Carestream Health based inRochester, NEW YORK (USA)
Capturing high-quality, long-length images of legs or spine can be difficult – especially with pediatric patients or patients with limited mobility who find it hard to remain still for an extended period of ...
Manufactured by:Neuro France Implants based inLa Ville aux Clercs, FRANCE
The PM BUTTERFLY® system is intended to provide osteosynthesis between two or more cervical vertebrae thanks to a plate and cervical ...
Manufactured by:Excite Medical Corp. based inTampa, FLORIDA (USA)
The DRX9000® True Non-Surgical Spinal Decompression Combination SystemTM is designed to provide pain relief for compressive and degenerative injuries of the spine. Through the application of spinal decompressive forces to these injuries, the DRX9000®TM has given patients relief from back pain or neck pain and has allowed them to resume the activities they ...
Manufactured by:Zealmax Innovations Pvt. Ltd. based inAhmedabad, INDIA
For degenerative cervical procedures, the Cervical DIO Cage system offers an optimized configuration solution. The graft’s large surface area allows for the appropriate use of ortho-biological material, which is designed to reduce the risk of ...
Manufactured by:AIDFLEX LTD based inObninsk, RUSSIA
Built-in verticalizer, power stations, unloading and slider systems provide for a wide range of work with different categories of patients suffering from ordinary musculoskeletal pain to progressing degenerative-arthrotropic and spine diseases as well as complex neurological diseases with loss of sensitivity and movement. ...
Manufactured by:Trulife based inTallaght, IRELAND
Lightweight heat moldable Kydex shell® with soft, closed-cell foam liner, Rigid support controls flexion, extension and rotation, Independently height-adjustable anterior and posterior sections, Trachea opening, Thoracic Extension available, CV102. Cervical spine precaution for trauma patients, immobilization for pre and post cervical spine surgery, ...
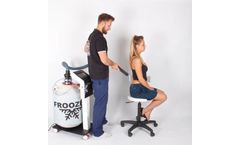
Manufactured by:Technomex based inGliwice, POLAND
Froozer device allows the local cooling of the body of the patient by contact with the cold vapor stream of nitrogen at -160 ° C. It is equipped with a touch screen for easy and fast operation. Cold nitrogen vapor stream is directed to the skin of the patient using a flexible. Liquid nitrogen is stored in a special 30-liter container. Device can be upgraded with a replacement bottle, allowing ...
Manufactured by:Orthofix Medical Inc. based inLewisville, TEXAS (USA)
The Centurion Posterior Occipital Cervico-Thoracic (POCT) System is a temporary, multiple component system comprised of a variety of non-sterile, single use components made of titanium alloy or cobalt chrome alloy that allow the surgeon to build a spinal implant construct. The Centurion POCT System consists of an assortment of rods, set screws, axial connectors, lateral offset adapters, ...
Manufactured by:Ankasa Regenerative Therapeutics based inLa Jolla, CALIFORNIA (USA)
Stem cell renewal and maintenance and tissue regeneration are critical for normal health and well-being. Wnt pathway interactions are crucial to maintenance of these processes. Ankasa is the first to produce human Wnt proteins in a manufacturing setting suitable for use in humans. Our first clinical approach will be the demonstration of improved outcomes after spinal fusion surgeries. Our product ...
Manufactured by:Noraker based inLyon, FRANCE
Inspired by an innovative and reliable technology, Glassbone injectable putty is indicated in orthopedic CMF and spine ...