Show results for
Refine by
Drug Development Equipment Supplied In Sweden
10 equipment items found
Manufactured by:Gesynta Pharma AB based inStockholm, SWEDEN
The drug candidate GS-659, which is sprung from Gesynta Pharma’s drug development platform, is currently in preclinical development phase. Project updates will be announced in due ...
Manufactured by:SHL Medical AG based inZug, SWITZERLAND
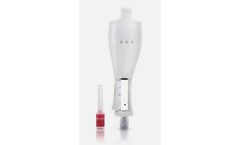
The development of the Precision Pen Injector (PPI) is a story of uncompromising dedication to meeting industry challenges. A Swedish company specializing in solutions for aesthetics and corrective treatment sought to introduce an autoinjector for its skin booster – a highly viscous hyaluronic acid formulation of several hundred centipoise. The company needed a device that could deliver ...
Manufactured by:Recipharm AB based inStockholm, SWEDEN
Recipharm offers a range of inhalation manufacturing and development capabilities for novel respiratory products. Our modern, well equipped facility in Holmes Chapel offers manufacturing, packaging and warehousing facilities for inhalers and nasal sprays, as well as end-to-end quality control, GMP compliancy and IMP licence for clinical ...
Manufactured by:2A Pharma ApS based inAalborg Øst, DENMARK
2A Pharma’s anti-amyloid-beta vaccine candidate, AAVLP-Amyloid-β, has in laboratory tests with transgenic mice over-expressing amyloid-beta mutant protein been shown to eliminate amyloid-beta soluble protein, a clear plaque from the cortex and significantly reduce plaque from the hippocampus. It is our hope that the drug candidate can be developed into ...
Manufactured by:Diamyd Medical AB based inStockholm, SWEDEN
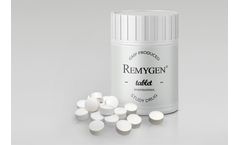
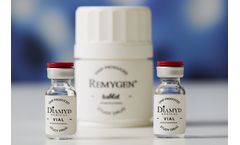
Remygen® is a controlled release tablet consisting of the active pharmaceutical ingredient GABA. The GABA substance is chemically synthesized in a well-controlled GMP process together with a global contract manufacturer. The manufacturing process is scalable and pediatric formulations with the same properties can be designed. ...
Manufactured by:Diamyd Medical AB based inStockholm, SWEDEN
Diamyd® is a suspension for injection containing the active pharmaceutical ingredient GAD65 mixed with the vaccine adjuvant Alhydrogel (alum). The human recombinant protein GAD65 is manufactured in insect cells in a well-controlled GMP process. The manufacturing process is developed for late phase clinical use. A new manufacturing facility is being set up by Diamyd Medical in Umeå, ...
Manufactured by:BioArctic AB based inStockholm, SWEDEN
In Alzheimer’s disease soluble, toxic amyloid beta aggregates are believed to contribute to the neurodegenerative process. Lecanemab, a anti amyloid-beta protofibril antibody, selectively binds to these forms of amyloid beta and eliminates them. The antibody’s unique profile is highly selective for amyloid beta oligomers/protofibrils in the brain. BioArctic’s belief is that ...
Manufactured by:ICU Medical, Inc. based inSan Clemente, CALIFORNIA (USA)
Maintain a needlefree closed system to help minimize exposure to hazardous drugs and comply with USP. Prohibits the escape of hazardous drug or vapor concentrations, Blocks the transfer of environmental contaminants into the system, Eliminates needlestick injuries while minimizing , hazardous drug exposure. With the ChemoLock needlefree CSTD, it has never been easier to keep yourself safe from ...
Manufactured by:Vivesto AB based inSolna, SWEDEN
Many intravenously delivered Active Pharmaceutical Ingredients (APIs) are insoluble or poorly soluble in water. This can be a major hurdle in pharmaceutical development and may cause promising drugs to fail during the development process and may limit the application of approved drugs because of poor solubility. According to some ...
Manufactured by:BioArctic AB based inStockholm, SWEDEN
BioArctic has developed a technology platform that has proven to be successful when the company’s first drug candidate was developed, the antibody BAN2401 for Alzheimer´s disease. Our mission is to improve quality of life for patients suffering from Central Nervous System (CNS) disorders. ...