Refine by
Drug Safety Equipment & Supplies
48 equipment items found
Manufactured by:InSphero AG based inSchlieren, SWITZERLAND
These advanced 3D liver models, which combine multi-donor diversity with structural and functional robustness, are ideal for drug safety and efficacy testing as well as the study of healthy and diseased liver function. Upon request, microtissues from individual donor hepatocyte lots are also ...
Manufactured by:Tissue Genesis based inNew Albany, INDIANA (USA)
Temperature controlled digestion and separation. Class III approval for commercial use by the South Korean Ministry of Food and Drug Safety (MFDS). Approved for clinical use in South Korea & The Bahamas. In the USA, FDA-approved clinical IDE studies using the Icellator and Cell Icellation Kit are underway for multiple indications. Produced under ISO 13485 ...
Manufactured by:West Pharmaceutical Services, Inc. based inExton, PENNSYLVANIA (USA)
Daikyo D Sigma components offer high-quality elastomer components for injectable medicines that are designed to reduce particulate, mitigate patient safety risks and reduce variability. A new level of innovation excellence, Daikyo D Sigma components combine advanced technologies and a long history of industry ...
Manufactured by:Links Medical Products Inc. based inIrvine, CALIFORNIA (USA)
The Silent Knight Hazardous Drug (HD) Safety Seal is a simple yet effective new product that is compliant to the USP 800 Standard of safe medication handling to minimize the risk of exposure to healthcare personnel, patients and the environment. The Silent Knight HD Safety Seal works together with the Silent Knight pouch and Silent Knight pill ...
Manufactured by:CN Bio based inCambridge, UNITED KINGDOM
NASH-in-a-box contains everything required to recreate our industry-validated human in vitro Non-alcoholic steatohepatitis (NASH) model in your own ...
Manufactured by:Taconic Biosciences, Inc. based inRensselaer, NEW YORK (USA)
Origin: Taconic received the Swiss Webster outbred model from the Rockefeller Institute through Rockland Farms, Inc. in 1940. The mice have been maintained as a closed colony since 1951. The mice were derived by caesarean from randomly chosen breeders and reintroduced into a Barrier Nucleus Expansion Colony in 1965-1969. The mice were derived by caesarean in 1983 from randomly chosen breeders ...
Manufactured by:Pharma Integration based inSiena, ITALY
Azzurra Small stands as a pioneering fully robotic monobloc system tailored for the pharmaceutical manufacturing industry. This advanced system incorporates four compact robotic modules, setting a benchmark in industrial-scale production, particularly designed for small-batch manufacturing of high-value pharmaceuticals. The system enhances safety, efficiency, and consistency through cutting-edge ...
Manufactured by:JW Holdings based inSeocho-gu, NORTH KOREA
Self-microemulsifying drug delivery system (SMEDDS) is a solubilization technology for improving bioavailability of poorly soluble drugs. By combining surfactant and co-surfactant with a poorly soluble drug, this technology improves the bioavailability of the drug by forming fine emulsion droplets upon dilution with physiological fluid after administration. This proprietary technology utilized ...
Manufactured by:Concept Medical Inc. based inTampa, FLORIDA (USA)
Erectile dysfunction (ED) is de?ned as the recurrent inability to achieve and maintain an erection satisfactory for sexual ...
by:Genmab A/S based inCopenhagen V, DENMARK
Daratumumab is a human IgG1k monoclonal antibody (mAb) that binds with high affinity to the CD38 molecule, which is highly expressed on the surface of multiple myeloma cells.1 Daratumumab is being developed by Janssen Biotech, Inc. under an exclusive worldwide license to develop, manufacture and commercialize daratumumab from Genmab. A subcutaneous formulation of daratumumab, DARZALEX ...
Manufactured by:Mediplus Ltd. based inHigh Wycombe, UNITED KINGDOM
Designed to ensure optimal drug delivery for enhanced safety in ICU, HDU, orthopaedic and obstetric ...
Manufactured by:InSphero AG based inSchlieren, SWITZERLAND
When you’re ready, we deliver. InSphero assay-ready spheroid microtissues are engineered and certified for use in critical drug development applications, and validated to ensure uniform size, robust functionality, and reproducible results. We develop 3D InSight™ Microtissues using the most advanced 3D cell culture technologies available to produce highly predictive, ...
Manufactured by:Concept Medical Inc. based inTampa, FLORIDA (USA)
A functioning dialysis access (AVF/AVG) is critical to the delivery of life saving hemodialysis therapy in patients with End Stage Renal Failure. PROMISING REVASCULARIZATION THERAPY FOR DYSFUNCTIONAL DIALYSIS FISTULA . MagicTouch AVF is intended for stenotic lesions of dysfunctional arteriovenous fistula and graft. ...
by:Hummingbird Diagnostics GmbH based inHeidelberg, GERMANY
Hummingbird’s powerful discovery engine generates biomarkers for diagnostics, drug discovery and safety. Collection of samples from world class clinical network. Preparation and measurement following robust SOPs - additional SOPs for all other major biofluids. Identification of signatures using unique bioinformatics platform. ...
Manufactured by:Pharma Integration based inSiena, ITALY
This system is crafted to optimize micro-batch production, making it suitable for diverse applications such as cell and gene therapies, clinical trials, and personalized medicine.It seamlessly accommodates a broad range of pharmaceuticals, from traditional compounds to complex biologics, especially large biological molecules.The system's robotic technology, utilizing Denso 6-axes robots, ensures ...
Manufactured by:HPF Hans P. Friedrich Elektronik GmbH based inUrbach, GERMANY
The HPF EOK-TT Track & Trace System is a robust and comprehensive solution crafted to enhance the traceability of pharmaceutical products throughout the entire distribution chain. Integrated within the packaging process, this system ensures that each package is labeled with a data matrix code containing pertinent data that is archived in a tracking database. This technology not only bolsters ...
Manufactured by:Concept Medical Inc. based inTampa, FLORIDA (USA)
AN EXCELLENT ALTERNATIVE FOR REVASCULAZATION OF SFA AND BTK ARTERIES.MagicTouch PTA is intended to prevent re-narrowing of superficial femoral and popliteal arteries in patients with peripheral artery ...
Manufactured by:Concept Medical Inc. based inTampa, FLORIDA (USA)
Novel, First of its kind Sirolimus drug coated balloon catheter for the treatment coronary artery disease. MagicTouch is intended for in-stent restenosis, small vessels, bifurcation lesions and de-novo lesions ...
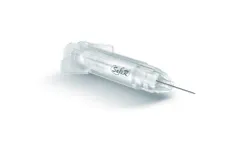
Manufactured by:Roncadelle Operations Srl based inCastel Mella (BS), ITALY
The SafeR Shield is an advanced safety mechanism designed for pre-filled syringes, ensuring the needle is entirely encased upon administration completion. This feature eliminates the risk of needle-stick injuries and minimizes healthcare-associated infections. Uniquely, since the drug within the syringe is isolated solely within the syringe throughout its shelf ...
Manufactured by:Orchestra BioMed, Inc. based inNew Hope, PENNSYLVANIA (USA)
Virtue SAB is a patented drug/device combination product for the treatment of artery disease that delivers a proprietary extended release formulation of sirolimus, SirolimusEFR, during balloon angioplasty without the need for a coating or a permanent ...