- Home
- Equipment
- north america
- immunotherapeutic
Show results for
Refine by
Immunotherapeutic Equipment Supplied In North America
137 equipment items found
by:Sumitomo Pharma Oncology, Inc. based inCambridge, MASSACHUSETTS (USA)
Immunotherapeutic cancer vaccines have generated a lot of interest from oncologists since it was discovered that immune activation can lead to a specific antitumor response.1 However, despite initial progress, most cancer vaccines have failed to drive sufficient tumor immunogenicity, leading to poor clinical responses.1 The reason for this may be that previous vaccine models used ...
Manufactured by:Altimmune Inc. based inGaithersburg, MARYLAND (USA)
An immunotherapeutic product candidate for patients chronically infected with the hepatitis B virus (HBV). It is designed to drive CD4+ and CD8+ T-cell responses against all HBV genotypes in patients of diverse genetic backgrounds. Normally, the HBV virus is eliminated through T cell dependent mechanisms but stimulating these responses in chronically infected HBV ...
by:Sumitomo Pharma Oncology, Inc. based inCambridge, MASSACHUSETTS (USA)
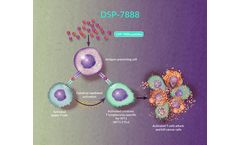
Ombipepimut-S Emulsion (DSP-7888) is an investigational immunotherapeutic cancer vaccine containing 2 peptides that induce WT1-specific cytotoxic T lymphocytes (WT1-CTLs) and helper T cells to attack WT1-expressing cancerous cells found in various types of hematologic malignancies and solid tumors.1,2 Researchers have identified that adding helper T-cell–inducing peptides ...
Manufactured by:Allovate Therapeutics based inNew York City, NEW YORK (USA)
Allovate’s approach to allergy immunotherapy is built around the Allerdent® allergy therapeutic platform. The proprietary Allerdent® toothpaste base is designed to incorporate and stabilize immunotherapeutic agents used to treat allergies. The Allerdent® toothpaste base is available without active pharmaceutical agents. It can be customized by physicians to ...
Manufactured by:Brooklyn ImmunoTherapeutics (BTX) based inSan Diego, CALIFORNIA (USA)
The gene-edited iPSC will then be used to produce iMSC that have been endowed with beneficial properties to broaden and enhance therapeutic uses. Our first gene-edited iMSC product will be used as a cancer immunotherapeutic, taking advantage of the tumor homing properties of MSC and engineering the cells to deliver immune-stimulating proteins to enhance the immune system’s ...
Manufactured by:BriaCell Therapeutics Corp. based inWest Vancouver, BRITISH COLUMBIA (CANADA)
This target has an attractive safety profile based on in vivo studies and knock out mouse studies. PKCδ also has potential activity as an immunotherapeutic by blocking TGFβ signaling. PKCδ inhibitors are applicable to specific niche tumor types which provide an accelerated clinical development plan. Could be in clinic within 24 months. Provides Cost-Effective ...
Manufactured by:IDT Biologika based inDessau-Rosslau, GERMANY
IDT Biologika is a leading viral vector CDMO with an exceptional track record in biologics manufacturing, including expertise with live viral agents. This makes us well-positioned to support the rapid growth in gene therapy and immunotherapeutics markets and the huge demand for transformative viral ...
Manufactured by:Allovate Therapeutics based inNew York City, NEW YORK (USA)
Immunotherapy has a 100-year track record of success for respiratory allergies. OMIT improves upon this approach by delivering immunotherapeutic agents to the areas of the oral cavity with the highest likelihood of prompting a decrease in allergy symptoms in order to improve the health of allergy sufferers. Clinical investigations led by Allovate are developing the OMIT platform ...
Manufactured by:Brooklyn ImmunoTherapeutics (BTX) based inSan Diego, CALIFORNIA (USA)
Why human derived? Brooklyn has developed a powerful method to create complex cytokine mixtures derived from a human source. Cytokines are powerful immune factors that play an essential role in T cell signaling, activation, proliferation, and tumor-fighting responses. We know cytokines may induce beneficial reactions in cancer patients. To date, available cytokine therapies have been recombinant ...
Manufactured by:Brooklyn ImmunoTherapeutics (BTX) based inSan Diego, CALIFORNIA (USA)
IRX-2 is a human-derived mixed cytokine product containing more than 29 cytokines that promote or enhance a complex immune response. Scientists are developing IRX-2 as a cancer immunotherapy. IRX-2 is administered as a subcutaneous injection around lymph node beds near the tumor target. IRX-2 is produced under cGMP (current good manufacturing process) conditions following stimulation of a ...
Manufactured by:Brooklyn ImmunoTherapeutics (BTX) based inSan Diego, CALIFORNIA (USA)
Messenger RNA (“mRNA”) is a special class of molecules containing instructions that determine how cells function. Brooklyn’s platform is being designed to harness mRNA to engineer cells to treat disease by repairing disease-causing mutations and directing the formation of stem cells. Expressing reprogramming proteins by repeated transfection with protein-encoding RNA could ...
Manufactured by:Brooklyn ImmunoTherapeutics (BTX) based inSan Diego, CALIFORNIA (USA)
Mesenchymal stem/stromal cells (MSC) have undergone extensive clinical testing for many diseases and have consistently demonstrated safety. In addition, the immunomodulatory properties of MSC have been well characterized. However, adult-tissue-derived MSC have shown inconsistent therapeutic efficacy, significant variability among samples, and limited proliferative capacity. On the other ...
Manufactured by:Brooklyn ImmunoTherapeutics (BTX) based inSan Diego, CALIFORNIA (USA)
Our partners at Factor Bioscience have developed a technology that uses mRNA to express gene-editing proteins. This technology can enable dramatically higher efficiency gene editing, including in primary cells, than other approaches, without using viruses or DNA-based vectors that may cause unwanted mutagenesis. We can use this technology to inactivate one or more genes and/or insert a donor ...
Manufactured by:Brooklyn ImmunoTherapeutics (BTX) based inSan Diego, CALIFORNIA (USA)
The foundational technology of synthetic mRNA, precise gene-editing machinery, and non-viral delivery also enable the development of genetic medicines with curative potential for multiple disease states. Brooklyn is developing products designed to edit a patient’s genome inside their body and address diseases with clear genetic causation. ...
Manufactured by:Brooklyn ImmunoTherapeutics (BTX) based inSan Diego, CALIFORNIA (USA)
Cellular reprogramming refers to the process of transforming patient skin cells into pluripotent stem cells, called induced pluripotent stem cells (iPSC). Since 2012 Nobel Prize Winner Dr. Shinya Yamanaka's initial discovery of iPSC in 2006, iPSC availability has transformed the field of cell therapy. Brooklyn uses the fastest, highest-efficiency method for generating iPSC – a key ...
Manufactured by:Immune Biosolutions Inc. based inSherbrooke, QUEBEC (CANADA)
Leveraging our technology platforms, we hack the immune system of chickens to discover and engineer the next generation of immunotherapies. ...
by:Metaclipse Therapeutics Corporation based inAtlanta, GEORGIA (US) (USA)
Influenza (Target age 65+) and Influenza+ SARS-C0V-2 ...
Manufactured by:BioFactura, Inc. based inFrederick, MARYLAND (USA)
BioFactura is developing a Marburg therapeutic in close collaboration with the United States Army Medical Research Institute of Infectious Diseases (USAMRIID). Marburg virus (MARV) was first described in 1967 as the causative agent of a hemorrhagic fever illness that occurred in laboratory workers at a primate facility in Germany and Yugoslavia. Since its discovery, marburgviruses have caused at ...
Manufactured by:Transgene based inIllkirch-Graffenstaden, FRANCE
A particularly innovative immunotherapy, TG4050 is the lead myvac® candidate. This individualized treatment is based on the specific mutations that are identified in each patient’s ...
by:Sorrento Therapeutics, Inc. based inSan Diego, CALIFORNIA (USA)
Sorrento utilizes a proprietary knock-out knock-in (KOKI) technology to modify normal healthy donor derived T cells to genetically engineer them to express the dimeric antigen receptor into T-cell receptor (TCR) alpha chain constant region (TRAC). In this manner, TRAC is knocked out and antigen is knocked into its locus. The Dimeric Antigen Receptor (DAR) utilizes a Fab instead of the scFv ...