Show results for
Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
Implant Bar Specifications Equipment Supplied In Europe
30 equipment items found
Manufactured by:Spiggle & Theis Medizintechnik GmbH based inOverath, GERMANY
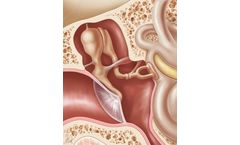
In otoplasty, middle ear implants serve as replacements for damaged or redundant ossicles. They restore the defect mechanical chain of sound transmission between the tympanum and the stapes ...
Manufactured by:Districlass Médical SA based inSAINT-ETIENNE, FRANCE
Model : 245, Matter : Titanium/Polysulfone, Weight (g) : 6 g, Height x base (mm) : 12 x 29,9, ø puncture (mm) : 13, Residual Volume (ml) : ...
by:Nelson Laboratories, LLC based inSalt Lake City, UTAH (USA)
Cytotoxicity: MEM elution, 48 hr inc., L929, 24 hr ext ...
by:Nelson Laboratories, LLC based inSalt Lake City, UTAH (USA)
Cytotoxicity: MEM elution, 48 hr inc., L929, 72 hr ext ...
Manufactured by:3di GmbH based inJena, GERMANY
We use the glass ceramics BIOVERIT II, which has been developed under the supervision of Prof. Vogel by the department of glass chemistry of the Friedrich-Schiller-University Jena. This material consists of machinable glass ceramics of the SiO2-Al2O3-MgO-Na2O-K2O-F-system. BIOVERIT®II is an non-resorbable glass ceramic, which consists of a crystal phase (approx. 60%) with mainly ...
Manufactured by:3di GmbH based inJena, GERMANY
PEEK was deverlop as non-resorbable high performance thermoplastic for the long-term implantation. Since 2008, 3di GmbH is producing patient specific implants for the cerebral and visceral cranium made of this material. PEEK is intraoperative ...
Manufactured by:3D Medical based inMarolles-en-Brie, FRANCE
Patient-specific mandible implant is designed on the patient’s skull-mandible anatomical model that is obtained from patient‘s CT scan ...
Manufactured by:Aleva Neurotherapeutics SA based inLausanne, SWITZERLAND
The directSTIM™ DBS System is a rechargeable system with 2 x 12-electrode lead. The System can be implanted to deliver unilateral or bilateral stimulation. For optional intraoperative testing during lead placement, a trial stimulator provides stimulation by emulating the implantable stimulator. The clinician sets the stimulation with the Clinician Programmer. The patient uses the Patient ...
Manufactured by:Conformis based inBillerica, MASSACHUSETTS (USA)
Engineered for low contact stress throughout the range of motion, Conformis knee replacement systems are designed to reduce the risk of polyethylene wear. We use the latest material technology for stronger and more durable polyethylene inserts, and offer two types of polyethylene material: iPoly, and iPoly ...
Manufactured by:Dieter Marquardt Medizintechnik GmbH based inSpaichingen, GERMANY
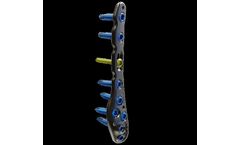
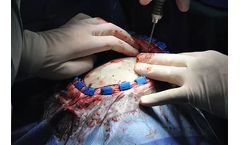
WINSTA-FiT – anatomical, polyaxial, locking. Universal plating system made of titanium. The WINSTA-FiT plating system by Marquardt Medizintechnik offers anatomically shaped plates that have been specially developed for locking plate fixation of fractures of the distal fibula and tibia. Focusing on the essentials, a clearly arranged instrumentation and full compatibility with the Marquardt ...
Manufactured by:Newclip Technics based inHaute Goulaine, FRANCE
The implants of the Alians Clavicle range are dedicated to the fixation of fractures, mal-unions, non-unions, and osteotomies of the clavicle in adults. Alians Clavicle S allows treating simple and complex fractures of the clavicle from the medial third to the lateral third by superior or anterior approach by proposing a complete range of ...
Manufactured by:Cousin Surgery based inWervicq-Sud, FRANCE
Double-sided parietal prosthesis with expansion balloon (as per reference). Repair and parietal reinforcement for umbilical, incisional and small ventral hernias. Intraperitoneal ...
Manufactured by:3D-Side based inMont-Saint-Guibert, BELGIUM
The device is a patient specific mold that can create a patient specific cranial implant from polymethylmethacrylate (PMMA, bone cement) during surgery. Latest technologies are used for the design (AI-based segmentation & unique platform for the surgeon) and production (high accuracy 3D printer) of this device allowing short leadtime. Based on the CT-scan of the patient, the mold is ...
Manufactured by:Cousin Surgery based inWervicq-Sud, FRANCE
Double sided implant for parietal reinforcement: one side made of monofilament polypropylene for tissue ingrowth and one side made of ePTFE to limit adhesions.Meshes are marked with a cross of biocompatible ink on the anti-adherent side. Repair and parietal reinforcement of umbilical and ventral hernias. Intra-peritoneal ...
Manufactured by:Hemodia SAS based inLabège, FRANCE
From the patient's preparation to the care: Syringes, needle packs; Specific implanted ports care kits dedicated to needle installation, infusion connection and disconnection; Chemotherapy decontamination kits: for carers handling cytotoxic agents. Our packs regroup all devices and consumables required for preparing and carrying out an intravenous chemotherapy session. To be totally in line with ...
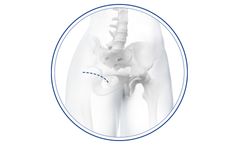
Manufactured by:Medacta International based inCastel San Pietro, SWITZERLAND
As part of the AMIS EXPERIENCE offer, the AMIS BIKINI represents an ADVANCED level of our anterior minimally invasive surgical offer that surgeons can experience within the comprehensive AMIS educational program. ...
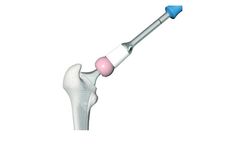
Manufactured by:GO German Orthopedic Implants GmbH based inHamburg, GERMANY
for total hip arthroplasty are small ceramic balls that are placed onto the hip stem. Due to the conical design of the 12/14 taper, the heads sit firmly on the stem and require no additional fixation. EasyHip Ceramic Heads come in three diameters (28 mm, 32 mm and 36 mm) and four head neck lengths, ranging from -4 mm to +8 mm. Detailed correlation can be seen in table ...
Manufactured by:INNOTERE GmbH based inRadebeul, GERMANY
INNOTERE offers comprehensive services for the manufacturing of patient-specific, apatitic implants to reconstruct or support individual bone defects. Based on the patient’s computed tomography data INNOTERE designs implants according to the requirements of the surgeon and anatomical needs of the defect site. 3D-printing of INNOTERE's innovative calcium phosphate bone cement paste and low ...
Manufactured by:Cousin Surgery based inWervicq-Sud, FRANCE
Inflatable expansion balloon parietal prosthesis, knitted monofilament polypropylene and poly-L-lactic acid (PLLA). The implant is semi-resorbable. Repair and parietal reinforcement of umbilical, incisional hernias and small eventrations. Extra-peritoneal ...
Manufactured by:Medacta International based inCastel San Pietro, SWITZERLAND
AMIS (Anterior Minimally Invasive Surgery) is the only hip replacement technique that follows both an inter muscular and inter nervous path to greatly decrease damage to periarticular structures while preserving tissue. AMIS is a true muscle-sparing procedure supported by the industry’s most detailed surgeon training protocol. ...