Show results for
Refine by
Implant Guided Surgical Equipment Supplied In Germany
22 equipment items found
Manufactured by:NDI Medical based inBad Emstal, GERMANY
Designed for proper formation of the bone socket for the implant. Made of surgical stainless steel 1.4197 (X20 CrNiMoS13-1) that has been specially sharpened. Tensile strength Rm: 881-900 N/mm². Superior corrosion resistance and wear resistance. Depth mark grooves at 8, 10, 11.5, 13, and 15 mm. Additional laser marking inside the ...
Manufactured by:GEUDER AG based inHeidelberg, GERMANY
Retinal implants are used in surgical treatment of retinal detachments. The implant creates an indentation in the sclera and choroid approximating the retina to the pigment epithelium. This procedure is accomplished by local indentation or through the use of an implant that encircles the ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Non-bone tendons are sterilized through the BioCleanse Tissue Sterilization Process without the use of irradiation. Non-bone tendons used in ACL reconstruction procedures allow for multiple fixation ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Matrix HD Allograft Dermis is an acellular human dermis allograft sterilized to a Sterility Assurance Level (SAL) of 10-6 using the Tutoplast Tissue Sterilization Process. This proprietary process retains the three-dimensional intertwined multidirectional fibers and mechanical properties of the native dermis tissue. The Matrix HD graft provides a natural scaffold to support the body's ...
Manufactured by:General Implants GmbH based inWurmlingen, GERMANY
General Implants surgical sets contain all the components needed for professionally preparing an implant procedure as well as the necessary instrumentation. Our practical racks for holding screws and plates make the treatment process straightforward, with colour-coding throughout to facilitate selection. There are no distractions, since everything is in the right place. Products and ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI's Achilles tendons are sterilized through the BioCleanse Tissue Sterilization Process without the use of irradiation. These tendons allow for multiple techniques and fixation options in ligament reconstruction ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Conventional allografts are used for various procedures to fill bony voids or gaps in a patient's skeletal system, restore segmental bone loss due to trauma, osteoporosis, removal of tumors or to aid in the surgical correction for a deformity. These grafts provide a scaffold for bone ingrowth to allow for remodeling with the patient’s own bone. These grafts are pre-cut to select sizes to ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Fortiva Porcine Dermis is a non-crosslinked acellular porcine dermal matrix offering a safe and natural biologic option. Fortiva implants are processed through the Tutoplast® Tissue Sterilization Process and terminally sterilized via validated low-dose gamma irradiation to achieve a sterility assurance level (SAL) of 10-6. Fortiva Porcine Dermis is a ready-to-use biologic implant that acts as ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI Surgical offers the only meniscal allograft with both bone and meniscus sterilized through the BioCleanse® Tissue Sterilization Process. The BioCleanse processed meniscus graft provides a sterilized alternative to traditional aseptically processed meniscus allografts and sets the standard for safety and ...
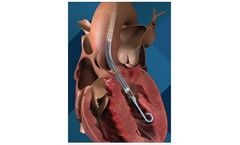
Manufactured by:Abiomed based inDanvers, MASSACHUSETTS (USA)
Impella 5.0 is a temporary, minimally invasive heart pump that provides circulatory support, enabling the heart to rest and recover. Impella LD is a surgically implanted heart pump that provides temporary support to the heart during and after a procedure. Impella 5.0 and Impella LD heart pumps allow patients to walk around while on support to improve recovery, if inserted via the axillary artery ...
Manufactured by:B. Braun Melsungen AG based inMelsungen, GERMANY
Askina Sorb is a sterile primary wound dressing made of fibers containing 85% of calcium alginate and 15% of carboxymethylcellulose (CMC). When in contact with wound exudate, the alginate-CMC fibers are fast gelling, resulting from an ionic exchange between calcium ions from the dressing and sodium ions from the exudate, so as to form a soft moist gel conducive to natural ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI's bone-tendon-bone (BTB) grafts are sterilized through the BioCleanse Tissue Sterilization Process without the use of irradiation. These grafts allow for multiple techniques and fixation options in ligament reconstruction ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Cortiva Allograft Dermis is a non-crosslinked acellular dermal matrix offering a safe and natural biologic option and sterilized to a sterility assurance level (SAL) of 10-6 through the Tutoplast® Tissue Sterilization ...
by:DOT GmbH based inRostock, GERMANY
An arc with high current density is run over the target material causing it to evaporate and to release titanium atoms, which are ionized and propelled towards the implant. During the process, nitrogen is introduced into the vacuum chamber. The TiN coating is formed when the titanium ions strike the implant surface and, simultaneously, combine with the nitrogen atoms. ...
by:DOT GmbH based inRostock, GERMANY
An arc with high current density is run over the target material causing it to evaporate and to release titanium and niobium atoms, which are ionized and propelled towards the implant. During the process, nitrogen is introduced into the vacuum chamber. The TiNbN coating is formed when the titanium and niobium ions strike the implant surface and, simultaneously, combine with the nitrogen ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Whole bones are used as a sterile source of cortical and cancellous bone. These implants may also be used in procedures that restore segmental and critical bone loss in the extremities due to trauma, bone disease, or from the removal of tumors and/or hardware. After being processed through RTI's BioCleanse® Tissue Sterilization Process, they are terminally sterilized in their final packaging ...
Manufactured by:MBP – Medical Biomaterial Products GmbH based inNeustadt-Glewe, GERMANY
Rematrix is a cell-free dermal collagen matrix derived from porcine dermis (ADM). Rematrix is intended for use as a surgical biomaterial implant for soft tissue repair, serving as a scaffold material, tissue coverage or tissue replacement. The double sterile packed membrane is available in various sizes. Rematrix is sold in dry form. It contains no ...
Manufactured by:BioCer Entwicklungs-GmbH based inBayreuth, GERMANY
TiO2Mesh™ is a surgical mesh implant specially indicated for repair of soft tissue defects of the abdominal wall, where a non-absorbable support material is required. Relevant applications include the repair of inguinal and incisional hernias in all common surgical procedures and even IPOM. Federal law (USA) restricts this device to sale by or on the order of ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI Surgical’s fresh-stored osteochondral (OC) allografts enable surgeons to resurface osteochondral defects with mature hyaline cartilage and repair subchondral bone in a single procedure. Fresh grafts are cleansed, processed and preserved to maintain chondrocyte ...
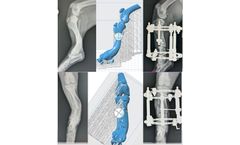
by:STERIS based inMentor, OHIO (USA)
STERIS Animal Health offers 3D Bone Prints in various materials including autoclavable and biocompatible plastics for deformity visualization, surgical rehearsal, and implant preparation. Deformity Visualization -Enhance understanding of a deformity by creating an exact replica used to better visualize the anatomy. Surgical Rehearsal – Cut, drill and apply implants to assist in surgical ...