Show results for
Refine by
Implanted Equipment Supplied In Ireland
27 equipment items found
Manufactured by:Spinal Stabilization Technologies, LLC based inKilkenny City, IRELAND
The PerQdisc Implant Delivery Device (Figure 4) is then inserted through the Access Cannula into the enucleated disc space. Contrast is injected into the inner chamber of the implant. This aids in visualization as well as provides real-time monitoring of fill pressure. The outer chamber of the implant is then filled under pressure control with a ...
Manufactured by:S3 Connected Health based inDublin, IRELAND
Our team understand the challenges scale-up medtech companies face when commercializing a medical device; we’ve taken many clients successfully through development and we can do the same for you. Using certified tools and processes, our experts will address performance and regulatory gaps and efficiently turn your prototype into a marketable product. ...
by:Electronic Recycling based inFinglas, IRELAND
Electronic Recycling handles all types of Electronic Equipment, Office, Commercial and Domestic, listed in The EU, Waste from Electrical and Electronic Equipment (WEEE) ...
Manufactured by:Aran Biomedical Teoranta based inSpiddal, IRELAND
Elastomeric coatings offer numerous functional benefits for implantable devices. The most obvious application is to create a blood impermeable barrier, which restricts blood flow to key locations. The barrier can also eliminate tissue adhesion to an implant, or contain material, such as thrombus, emboli or ureteral stone fragments, for example. ...
Manufactured by:Aran Biomedical Teoranta based inSpiddal, IRELAND
Hydrophilic coatings are used by Aran Biomedical to create a lubricious surface for implantable devices. The principle benefit of a lubricious coating is to substantially reduce surface friction, facilitating implant delivery and deployment, while additional benefits may be recognised in-vivo, such as reduced potential for tissue damage, lower bacterial ...
Manufactured by:Paragon 28, Inc. based inEnglewood, COLORADO (USA)
TenoTac® is a patent-pending procedure and soft tissue fixation system designed to permanently address the source of the toe contracture. Cannulated instrumentation allows for accurate and reproducible placement of the implant. A threaded titanium implant with a spiked base is used to achieve and hold soft tissue fixation. The procedure requires minimal steps ...
Manufactured by:Aran Biomedical Teoranta based inSpiddal, IRELAND
Whether braiding, knitting or weaving, Aran Biomedical has significant expertise manufacturing absorbable implantable textiles, such as absorbable mesh for hernia repair, absorbable sutures for sports medicine applications or implantable textiles for cardiovascular ...
Manufactured by:Aran Biomedical Teoranta based inSpiddal, IRELAND
Specifically, knitted fabrics offer compliance and the ability to negate unravelling of yarns. Where a knitted fabric is implanted can dictate particular compliance characteristics, to ensure that the material stretches or conforms with the movement of the body, limiting unwanted stresses or discomfort. Aran Biomedical uses digitally controlled equipment to precisely define ...
Manufactured by:Aran Biomedical Teoranta based inSpiddal, IRELAND
The weft yarns run transversely across the loom, with the warp yarn running longitudinally, creating a dimensionally stable implantable fabric. This implantable fabric configuration results in a flexible material with desirable performance attributes applied, such as high tensile strength, high suture retention, and a high level of burst strength. When compared ...
Manufactured by:Aran Biomedical Teoranta based inSpiddal, IRELAND
PTFE is a well-established biomaterial with a long clinical history of implantation. It has been approved by regulatory bodies for several decades and is used extensively in a range of vascular ...
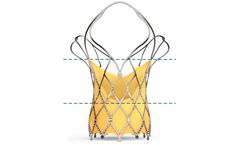
Manufactured by:Sahajanand Medical Technologies Ltd. based inSurat, INDIA
Self-expanding TAVI device to have 2 rows of marker; First Markers are located at Node 1. Second Markers are located at Node 3. First marker helps; In precise implantation of the valve at the targeted implantation zone. To ascertain the depth of implant. Second marker indicates; When the THV leaflets are going to get ...
Manufactured by:Aran Biomedical Teoranta based inSpiddal, IRELAND
Polyethylene terephthalate (PET) is the most common resin of the Polyester family, and the fibre is often referred to as Dacron or Terylene in the medical device industry. All our PET is implantable grade, and we work with monofilament and multifilament input yarns to develop low-profile braided constructs for implantable use. The braids can be supplied as ...
Manufactured by:Sahajanand Medical Technologies Ltd. based inSurat, INDIA
Hat-shaped design ensures secured implantation at the aorta and minimizes risk of migration into pulmonary artery. Unique Platinum coating. Device is filled with polypropylene fabric to assist thrombogenicity. Extended retention portion provides proper positioning of the device in the ductus arteriosus. 100% occlusion rate at one-month follow-up. ...
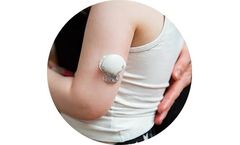
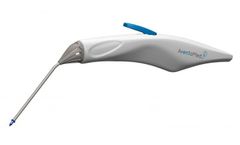
Manufactured by:AventaMed DAC based inCork, IRELAND
Solo+ TTD is an innovative, hand-held device which provides a new procedure for ENT surgeons. Solo+ TTD allows the ENT surgeon to implant grommets (ear tubes) outside the operating room using as little as topical anesthesia. ...
Manufactured by:Aran Biomedical Teoranta based inSpiddal, IRELAND
Aran Biomedical offers substrate micro fine wire for implantable devices. These high-density braids are made from ultra-fine wire and polymers and can be as low as 0.001” (25 micron). This low-profile wire braiding capability enables the production of fabrics with miniscule pore sizes, necessary for next generation braided cardiovascular devices. ...
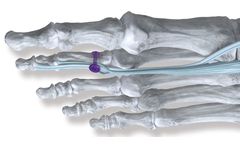
Manufactured by:Paragon 28, Inc. based inEnglewood, COLORADO (USA)
The Paragon 28 APEX 3D Total Ankle Replacement System was designed to address end-stage ankle arthritis and current challenges within the total ankle market including: implant loosening, pathological wear, instability and persistent pain. The APEX 3D System has been efficiently streamlined to accommodate surgeon preference and is equipped to address a wide range of arthritic ...
Manufactured by:Biomerics based inSalt Lake City, UTAH (USA)
We’ve worked with customers on steerable balloon catheters, steerable neurovascular devices, and delivery devices for structural heart implants, to name a few. As your vertically integrated partner, we have the end-to-end services to guide you through the entire product development ...
Manufactured by:Paragon 28, Inc. based inEnglewood, COLORADO (USA)
The JAWS™ Nitinol Staple System uses superelastic nitinol and a simple insertion method to gain rigid compression across an osteotomy site. Unlike many competitive implants, the JAWS™ Nitinol Staple System has a lightweight titanium inserter that allows the surgeon to fully seat the staple before it is released from the inserter. This allows for final placement of the ...
Manufactured by:Endotronix, Inc. based inLisle, ILLINOIS (USA)
Introducing the Cordelia® System, a solution that helps you achieve guideline-directed medical therapy with your patients using a streamlined workflow. Along with regular clinic visits, the system uses an intuitive Patient Management Portal and an at-home patient kit to improve outcomes and proactively treat more ...
Manufactured by:Paragon 28, Inc. based inEnglewood, COLORADO (USA)
The TITAN 3-D™ Wedge System offers a porous titanium wedge that provides an alternative to allograft/autograft bone for an Evans Calcaneal Osteotomy for lateral column lengthening. The TITAN 3-D™ Wedge System builds on Paragon 28®’s portfolio of osteotomy wedges and uses the same patented shapes as the PRESERVE™ Evans Wedges. ...