- Home
- Equipment
- usa arizona
- implanted
Show results for
Refine by
Implanted Equipment Supplied In Usa Arizona
17 equipment items found
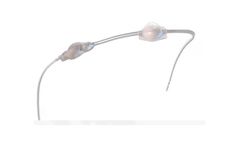
Manufactured by:Telonics, Inc. based inMesa, ARIZONA (USA)
Estimates of battery life are based on 250mW, 4-byte Argos transmissions with a 60-second repetition rate at 25°C. Argos transmissions are typically duty-cycled for an average of closer to the 4-hours/day example. Dimensions are exclusive of the antenna. ...
Manufactured by:Additive Implants LLC based inPhoenix, ARIZONA (USA)
SureMAX-XTM cervical spacer: The Advantages of Titanium with the Stiffness of PEEK. Large Lateral Windows to Allow Radiographic Confirmation of Fusion. Patented Lobe Design Allows for More Contact with Spinal End ...
Manufactured by:SynCardia Systems, LLC, based in, ARIZONA (USA)
The SynCardia 70cc temporary Total Artificial Heart (TAH) is the world’s first and only commercially approved total heart replacement. With more than 1,700 implants worldwide, the 70cc TAH accounts for more than 600 patient-years of ...
Manufactured by:SynCardia Systems, LLC, based in, ARIZONA (USA)
The state-of-the-art Companion 2 (C2) Hospital Driver provides pneumatic power to the SynCardia temporary Total Artificial Heart (TAH) from implant through patient recovery in the hospital. The C2 Driver monitors the TAH without the use of electrical connections to or inside the patient. Once the patient is clinically stable, he or she can be switched to the smaller, lighter ...
Manufactured by:Additive Implants LLC based inPhoenix, ARIZONA (USA)
SureMAX Cervical Spacer: Delivering Greater Stability at the Bone Implant Interface. Convex Surfaces to Match Spinal End Plates. Roughened and Optimized Porus Surfaces to Allow On-Growth and ...
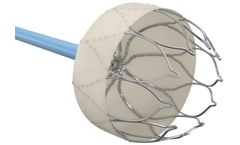
Manufactured by:Confluent Medical Technologies based inScottsdale, ARIZONA (USA)
Inclusions can be point of fracture initiation if located at a high strain area of the component. Ideal for high fatigue applications such as Cardiovascular Implants, or small feature components such as Neurovascular Stents. CT Scan images show Nitinol Tubing Cross Section comparison of SE508 (ASTM Standard Inclusion Rate) vs SE508-ELI (High Purity ...
Manufactured by:International Polymer Engineering, Inc. (IPE) based inTempe, ARIZONA (USA)
FluoroFlex™ ePTFE VS® suture components are manufactured in continuous lengths for suturing or custom applications. They are the ideal suture components for many implant surgical procedures where low elongation and high strength are required. Custom variations of the FluoroFlex™ ePTFE suture components can be developed upon ...
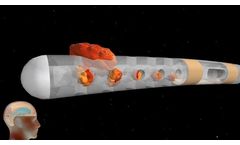
Manufactured by:Anuncia Medical, Inc. based inScottsdale, ARIZONA (USA)
The ReFlow™ System Mini offers the only noninvasive way to attempt to restore, increase, or maintain CSF flow within a shunt system. The ReFlow™ System Mini or the ReFlow™ Mini Flusher may be implanted as part of a shunt system in patients with hydrocephalus or conditions that require draining or shunting the CSF. Without surgery, pressing the ReFlow™ Mini ...
by:Align Technology, Inc. based inTempe, ARIZONA (USA)
iTero intraoral scanners are designed to deliver speed, reliability, intuitive operations, and outstanding visualization capabilities for general practitioners or orthodontists. iTero scans have been used in more than 8.4 million restorative crowns, bridges, and custom implant abutment cases and more than 40.1 million iTero orthodontic scans, for a total of 48.5 million ...
Manufactured by:Anuncia Medical, Inc. based inScottsdale, ARIZONA (USA)
The ReFlow System helps patients with cerebrospinal fluid (CSF) disorders, such as hydrocephalus, that require draining or shunting the CSF. The ReFlow Flusher connects directly to the Ventricular Catheter. Both are implanted under the scalp and connected to a shunt regulating valve (not included). The ReFlow System works with valves (programmable and non-programmable) and ...
Manufactured by:BCR Diagnostics Inc based inPhoenix, ARIZONA (USA)
Government guidelines require that a spore test should be used on each sterilizer at least weekly. A spore test should also be used for every load with an implantable device. It has become common practice to test sterilizer daily, but there is a trend to use BIs with each load. In this respect, an inexpensive BI like the BCR-30 min BI affords testing with each ...
Manufactured by:Confluent Medical Technologies based inScottsdale, ARIZONA (USA)
Confluent’s Biomedical Textiles service the Cardiovascular, Orthopedic, and Tissue Engineering markets. Woven, Knits, Braided, and Non-Woven implantable medical textiles are building blocks for medical devices. Trust our proven textiles expertise to assist you from initial concept development through large-scale ...
Manufactured by:Additive Implants LLC based inPhoenix, ARIZONA (USA)
Unmatched Options of Sizes, Lordotic Angles, Anchors and Easy to Use Instruments. Unique Designs to Maximize Stability and Reduce Rotation and Flexion. Fixed, Variable and Helical Anchor Options. One Set Instrumentation for all Three ...
Manufactured by:SynCardia Systems, LLC, based in, ARIZONA (USA)
The SynCardia 50cc temporary Total Artificial Heart (TAH) is a smaller version of the SynCardia 70cc TAH, which has been approved as a bridge to transplant for more than a decade. The 50cc TAH is designed for use as a bridge to transplant in patients of smaller stature, allowing more women and adolescents to access this novel ...
Manufactured by:W. L. Gore & Associates, Inc. based inNewark, DELAWARE (USA)
The GORE ACUSEAL Cardiovascular Patch is a high strength, low thrombotic, synthetic patch designed to decrease suture line bleeding in cardiovascular repair and reconstruction ...
Manufactured by:W. L. Gore & Associates, Inc. based inNewark, DELAWARE (USA)
The GORE PROPATEN Vascular Graft configured for Pediatric Shunt is the graft of choice by surgeons worldwide for palliative pediatric cardiac ...
Manufactured by:Confluent Medical Technologies based inScottsdale, ARIZONA (USA)
Sterrable Ablation Catheters: Through our proven engineering and manufacturing of catheters used in cardiac ablation, our partners trust us to design and produce femoral, transseptal, jugular, and subclavian catheters to treat arrhythmia-causing ...