Refine by
Implants Peek Medical Devices Equipment & Supplies
38 equipment items found
Manufactured by:Genesis Medical Plastics based inCypress, TEXAS (USA)
As one of the most complicated structures in the human body, the spinal column must be handled carefully, and with an eye for the future. Surgical intervention to correct conditions like degenerative disc disease (DDD), lumbar spinal stenosis, degenerative scoliosis or degenerative spondylolisthesis is a challenge, and the risks can escalate with time and when additional surgery is required. As ...
Manufactured by:LUXILON Industries nv | Medical based inWIJNEGEM, BELGIUM
Luxilon manufactures also materials that can be detected via radiotherapy (e.g. X-ray). The yarns contain BaSO4 which is visible under X-ray. By using these radio-opaque materials it is possible to detect, identify and control an implanted medical device. Luxilon offers radio-opaque monofilament yarns in PP and PET. The polymers used are the same as for Sterimed and Sterilux. The BaSO4 is medical ...
Manufactured by:Neuspera Medical, Inc. based inSan Jose, CALIFORNIA (USA)
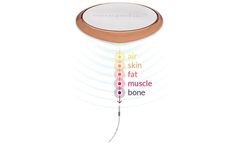
Neuspera’s patented Mid-Field Powering technology uses evanescent and propagating electromagnetic waves to power implanted medical devices to depths of 10cm and beyond. Using the body as a natural waveguide, the Neuspera system can focus power, ensuring energy is delivered to the precise area it is needed. Leveraging efficient power transfer, the power receiver is less than a few ...
Manufactured by:S3 Connected Health based inDublin, IRELAND
Our team understand the challenges scale-up medtech companies face when commercializing a medical device; we’ve taken many clients successfully through development and we can do the same for you. Using certified tools and processes, our experts will address performance and regulatory gaps and efficiently turn your prototype into a marketable product. ...
Manufactured by:Deringer-Ney Inc. based inBloomfield, CONNECTICUT (USA)
Separable connectors that require a high level of performance in adverse environments rely on Deringer-Ney materials and manufacturing. Pin-and-socket, pin-on-flat, and cantilever beam are the most common connector configurations for us. These can be found in specialty cable and plug connections for aerospace, defense, medical, and adverse industrial environments. Our contacts offer durability ...
Manufactured by:Valtronic Technologies (Suisse) SA based inLes Charbonnieres, SWITZERLAND
Valtronic was approached to design and manufacture the PCB for a programmable Morphine and Baclofen delivery pump. ...
Manufactured by:3D Systems based inRock Hill, SOUTH CAROLINA (USA)
Titanium alloy with high strength, low weight and excellent biocompatibility for technical and medical applications. Similar to laserform ti gr23 (a), but with higher strength. ...
Manufactured by:Peek China Co., Ltd based inTaizhou, CHINA
Medical-grade, biocompatible PEEK polymer materials Our wide range of biocompatible polymer materials and compounds are each designed to meet the specific and rigorous demands of implantable medical devices. PEEK materials are tailored to offer superior performance in a variety of applications ...
Manufactured by:Baoji Yinggao Metal Materials Co., Ltd. based inBaoji City, CHINA
High strength-to-weight ratio Excellent corrosion resistance Biocompatibility Heat ...
Manufactured by:Cirtec Medical based inBrooklyn Park, MINNESOTA (USA)
Vascotube, a Cirtec company, based in Birkenfeld, Germany, processes Nitinol tubing used in implantable medical devices including: Heart Valves, TAVR and TMVR Stents, Peripheral Vascular Stents, Neurovascular ...
Manufactured by:AccuPath Medical Technologies Co., Ltd. based inShanghai, CHINA
Medical nickel-titanium parts include conventional stents, valve stents, thrombectomy stents, close-mesh structural parts, and other parts. Medical push rods include (springs, tubes, and mandrels) welding structural push rods, spiral cutting structural push rods, and other parts. All these parts are widely used in various medical fields, such as the cardiovascular field, neural intervention, ...
Manufactured by:CorneaGen based inSeattle, WASHINGTON (USA)
Intacs Corneal Implants are an ophthalmic medical device designed for the reduction or elimination of myopia and astigmatism in patients with keratoconus so that their functional vision may be restored and the need for a corneal transplant procedure can potentially be ...
Manufactured by:Vascudyne, Inc. based inStillwater, MINNESOTA (USA)
Our proprietary TRUE Tissue technology platform is currently available for investigational use only. TRUE Tissue developed from cells isolated from donor tissue and is 100% biological. There are no synthetic materials or chemical fixation used, and implanted tissues are completely cell-derived and acellular. That’s how we make tissues that are TRUE to ...
Manufactured by:Mega Biopharma based inCrosne, FRANCE
Product / use: biological cements, watertight cements, for fixing the implant in place in the bone. Key properties: osteogenic, homeostatic, biocompatible, bioabsorbable, radiopaque, no volume reduction, no exothermic reaction. ...
Manufactured by:Novus Life Sciences based inFotan, HONG KONG
The Medical PMMA Filament by NOVUS stands out as a high-quality, biocompatible 3D printing material, characterized by its chemical resistance. It is specifically engineered for the creation of permanent implants, medical devices, and equipment, offering a reliable alternative to polycarbonate (PC) with enhanced transparency and a high-gloss finish. This filament is formulated from medical-grade ...
Manufactured by:Healionics based inSeattle, WASHINGTON (USA)
Healionics’ proprietary STAR (Sphere Templated Angiogenic Regeneration) biomaterial scaffold is a breakthrough in biointegration and tissue regeneration. It is the result of a sustained effort to discover the “sweet spot” – the exact pore size and structure that enables angiogenesis and healing while reducing Foreign Body Response. In contrast to many porous biomaterials ...
Manufactured by:Mega Biopharma based inCrosne, FRANCE
Product / use: plates, screws, rods, spacers, intramedullary nails. Key properties: osteogenic, homeostatic, biocompatible, bioerodible, radiopaque, no volume reduction, no exothermic reaction. ...
Manufactured by:Arrowhead Medical Device Technologies, LLC based inCollierville, TENNESSEE (USA)
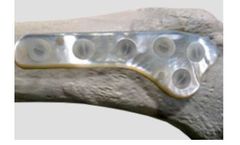
Design Rationale: In planning and research prior to design of the ARROW-LOK™ implant, the surgeon design team identified performance objectives for the new implant that would offer clinical advantages over current implant ...
Manufactured by:Valtronic Technologies (Suisse) SA based inLes Charbonnieres, SWITZERLAND
Deep brain stimulation (DBS) implant surgery has been found to give great improvement in motor function and to reduce symptoms of neurodegenerative diseases such as Parkinson’s. Our customer designed a directional stimulation probe which allows for targeted and controlled deep brain treatment. ...
Manufactured by:Mega Biopharma based inCrosne, FRANCE
Product / Use: injectable bone substitute for vertebroplasty and kyphoplasty. Key properties: osteogenic, homeostatic, biocompatible, bioabsorbable, radiopaque, no volume reduction, no exothermic reaction. ...