Refine by
In Vitro Diagnostic Equipment & Supplies
310 equipment items found
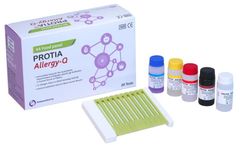
Manufactured by:PROTIA. iNC, formely known as ProteomeTech Inc. based inSeoul, SOUTH KOREA
PROTIA Allergy-Q 64S panel is an in vitro diagnostic test in the quantitative determination of allergen-specific IgE concentrations in human serum or plasma using immunoblotting technique. Diagnose 63 types of allergens including Total IgE in one ...
Manufactured by:PROTIA. iNC, formely known as ProteomeTech Inc. based inSeoul, SOUTH KOREA
GENEdania COVID-19 qRT-PCR is an in vitro diagnostic kit for the qualitative detection of COVID-19 (SARS-CoV-2) RNA in human upper and lower respiratory specimens using real-time reverse transcription polymerase chain reaction (qRT-PCR) ...
Manufactured by:PROTIA. iNC, formely known as ProteomeTech Inc. based inSeoul, SOUTH KOREA
ImmuneCheck IgG is an in vitro diagnostic kit for use in the semi-quantitative analysis of human IgG (hIgG) in human serum, plasma and whole blood samples using immunochromatography technology. " World's First" Human Immunoglobulin G Quantitative Analysis Rapid ...
Manufactured by:Omega Diagnostics Group PLC based inAlva, UNITED KINGDOM
VISITECT CD4 in-vitro diagnostic test is for use as an aid in the management of patients with pre-diagnosed HIV infection. This visually read test is designed to be used at the point-of-care and therefore has utility in decentralised diagnostic settings. ...
Manufactured by:Edif Instruments s.r.l. based inRoma, ITALY
The SARS-CoV-2 IgM ELISA kit is an in vitro diagnostic test for the qualitative detection of IgM antibodies to SARS-CoV-2 in human serum. The kit is intended for use as an aid in identifying individuals with an adaptive immune response to SARSCoV-2, indicating recent or prior infection. ...
Manufactured by:Abram Scientific, Inc. based inMenlo Park, CALIFORNIA (USA)
Less than 5-10 minutes from insertion of sample (approx. 20 microliters) to full viscoelastic curve and parameter generation, Low-cost, single-use diagnostic test strips that interface with a wireless, handheld meter that is vibration-insensitive, Mechanical viscoelasticity and density measurement for blood coagulation ...
by:Wi Inc based inEnglewood, COLORADO (USA)
Developing an IVD systems requires designing and developing both the hardware and the consumable. Wi possesses extensive experience developing complete IVD systems that incorporate complex electro-mechanical and optical hardware. Placing the development of both hardware and consumable at one location assures a seamless integration at the hardware-consumable interface. The hardware will actuate ...
Manufactured by:Avania based inBrunswick, AUSTRALIA
The right in vitro diagnostic (IVD) device can streamline clinical trials and expedite personalized patient care. We deliver world-class assistance for your IVD device development. We’ll help you build out effective research studies and protocols designed to validate algorithm reliability and ...
Manufactured by:Metafora Biosystems based inParis, FRANCE
METAglut1 is an in Vitro Diagnostic Medical Device used to aid in the diagnosis of the GLUT1 Deficiency Syndrome (GLUT1DS). It is a regulated health product that bears the CE mark. The first test for the daily practice developed from the METAdiag platform. ...
Manufactured by:iLine Microsystems S.L. based inDonostia, SPAIN
The microINR System is an in vitro diagnostics medical device, intended to monitor oral anticoagulation therapy (OAT) with vitamin K antagonist drugs. The microINR System refers to the reader (microINR Meter) and the analytic test strips (microINR Chips). Our system provides quantitative determination of prothrombin time (PT) in INR (International Normalized ...
Manufactured by:DxGen Corp. based inGunpo-si, SOUTH KOREA
Test Kit is for in-vitro diagnostic (IVD) use to determine C-reactive protein (CRP) quantitatively in serum/plasma, or whole blood from a human. It provides information for the diagnosis, therapy, and monitoring of inflammatory ...
Manufactured by:BMP Medical based inSterling, MASSACHUSETTS (USA)
BMP Medical has been producing components for In-Vitro Diagnostic (IVD) medical device testing for over 30 years. Many of the parts that we have developed with our customers have very intricate designs and features that set them apart from other products on the market. We work closely with our customers to provide support on the design and the ability to ...
Manufactured by:Cepheid based inSunnyvale, CALIFORNIA (USA)
Our GeneXpert family of systems have set a new standard in workflow flexibility, 24/7 testing accuracy, and user-friendly design — delivered in an astonishingly beautiful and compact package. The GeneXpert System is available in a 2, 4 or 16-module configuration. All have our proven GeneXpert module at their analytic heart, and use the same patented cartridge technology for every Xpert ...
Manufactured by:CLONIT srl based inAbbiategrasso, ITALY
DuplicαRealTime Next Factor V H1299R Kit is an in vitro diagnostic test for the detection of the nucleotide substitution A4070G of the blood coagulation Factor V gene, by Real-Time ...
Manufactured by:PROTIA. iNC, formely known as ProteomeTech Inc. based inSeoul, SOUTH KOREA
PROTIA Allergy-Q 128M panel is an in vitro diagnostic test in the quantitative determination of allergen-specific IgE concentrations in human serum or plasma using immunoblotting technique. Diagnose 118 types of allergens including Total IgE in one ...
Manufactured by:Werfen based inL`Hospitalet de Llobregat, SPAIN
Through a dedicated focus on autoimmune in vitro diagnostics and lab automation, we anticipate the needs of the world's most demanding laboratories and clinics. Our highly accurate reagents and automated systems are designed to serve labs of all sizes, and they help improve the way patients with autoimmune diseases are diagnosed, monitored, and treated around the ...
Manufactured by:Firalis Group based inHuningue, FRANCE
NEUROLINCS : A non-invasive in-vitro diagnostic test measuring 7494 brain-enriched long non-coding RNAs (lncRNAs) that are specific to neurodegenerative ...
Manufactured by:Abionic SA based inEpalinges, SWITZERLAND
Forcing molecules into a nanochannel, limiting the travel distance to a few hundred nanometres and reducing incubation time to 2 ...
Manufactured by:PROTIA. iNC, formely known as ProteomeTech Inc. based inSeoul, SOUTH KOREA
PROTIA Allergy-Q 64 Food panel is an in vitro diagnostic test in the quantitative determination of allergen-specific IgE concentrations in human serum or plasma using immunoblotting technique. Diagnose 72 types of food allergens including Total IgE in one ...
Manufactured by:Genomica, S.A.U. based inMadrid, SPAIN
CLART Fast PneumoVir is an in vitro diagnostic product for the detection and genotyping of the main viruses causing human respiratory infections, including the detection of Sars-CoV2. The technique used is based on our CLART® technology, and involves amplification of the sample by multiplex RT-PCR, and subsequent visualization on low density ...