Show results for
Refine by
In Vitro Release Testing Equipment & Supplies In Iran
85 equipment items found
Manufactured by:ACS Biotech based inVilleurbanne, FRANCE
Our strategy to combine the properties of chitosan (natural, biodegradable, biocompatible, cell adhesion) to a 3D hydrogel environment and selected biomolecules has led to develop a matrix (patented) whose performance and biocompatibility have been validated with in vitro tests on human cells in collaboration with the CNRS. ...
Manufactured by:DxGen Corp. based inGunpo-si, SOUTH KOREA
Epithod 616 D-Dimer Kit is an in vitro diagnostic (IVD) test for the rapid and quantitative measurement of the fibrin degradation product D-Dimer in the plasma. ...
Manufactured by:Immatics N.V. based inTuebingen, GERMANY
Immatics’ TCER molecules can be produced and purified utilizing established processes to manufacture ...
Manufactured by:Coris BioConcept based inGembloux, BELGIUM
In vitro rapid diagnostic test for the detection of SARS-CoV-2 antigen in nasopharyngeal secretions. Since many months, several variants did pop-up from countries where the pandemic was particularly active (i.e. UK, Brazil, India and South Africa) and our test has been proven to detect all of ...
Manufactured by:Xingtai Jiachuang Technology Co., Ltd. based inXingtai City, CHINA
Meister Supply Cosmetic Peptide Acetyl Tetrapeptide-9 Powder CAS 928006-50-2 Acetyl Tetrapeptide 9. Dermican is a pure synthetic tetrapeptide, singled out for its unique biological antiaging action targeted at increasing collagen network functionality via lumican synthesis. This newly identified tracer of aging is directly involved in the synthesis of collagen fibers and consequently in the ...
Manufactured by:PROTIA. iNC, formely known as ProteomeTech Inc. based inSeoul, SOUTH KOREA
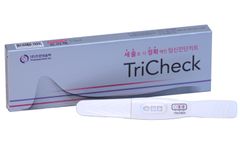
TriCheck, an in vitro self-testing kit, uses immunochromatography method for the qualitative test of hCG (human Chorionic Gonadotropin) in human urine samples to help the determination of ...
Manufactured by:MeKo Manufacturing e.K. based inSarstedt / Hannover, GERMANY
With RESOLOY MeKo offers a bioresorbable magnesium-alloy with extraordinary properties. RESOLOY is an attractive alternative to polymer scaffolds. In comparison to PLLA, it offers about 3 times higher strength, thinner struts, a higher radial force and is applicable for direct stenting. The degradation time of RESOLOY is adjusted by coatings. It has a proven biocompatibility, no shelf life and no ...
Manufactured by:ORGENTEC Diagnostika GmbH is part of the Sebia Group based inMainz, GERMANY
Anti-TG is an ELISA-based, automated, in-vitro test system for the quantitative determination of IgG autoantibodies against thyroglobulin (TG) in human serum or plasma. The protein thyroglobulin is responsible for the production and storage of thyroid hormones and is a strong autoantigen. B-lymphocytes with membrane-bound IgM antibodies against thyroglobulin are even detected in the blood of ...
Manufactured by:InActiv Blue bv based inBeernem, BELGIUM
stabilization of RNA/DNA at room temperature, inactivation of pathogens, mucolytic, clinical application: swab, saliva, can also be used as powerful lysis buffer, CE marked IVD. InActiv Blue® is a CE-IVD marked, virus inactivating and RNA stabilizing transport medium for swabs or saliva from patients for in vitro diagnostic testing by RT-qPCR or other molecular method (SARS-CoV-2, influenza ...
Manufactured by:ORGENTEC Diagnostika GmbH is part of the Sebia Group based inMainz, GERMANY
The assay contributes to the diagnosis of antiphospholipid syndrome (APS). The clinical symptoms of APS alone are not sufficiently specific to make a definitive diagnosis. Laboratory tests thus play an important role in the diagnosis of the disease. In patients with APS, autoantibodies are formed that bind to phospholipids like cardiolipin or to phospholipid-binding proteins like ...
Manufactured by:Lumiquick Diagnostics, Inc based inSanta Clara, CALIFORNIA (USA)
QuickProfile 2019-nCoV IgG/IgM Combo Test Card is an immunochromatography based one step in vitro test. It is designed for the rapid qualitative determination of IgG and IgM antibodies to 2019 novel coronavirus (2019-nCoV, SARS-CoV-2) in human serum, plasma, or whole blood. Rapid 2019-nCoV IgG/IgM Combo Test Card is a supplemental rapid screening tool for symptomatic or asymptomatic carriers of ...
Manufactured by:PROTIA. iNC, formely known as ProteomeTech Inc. based inSeoul, SOUTH KOREA
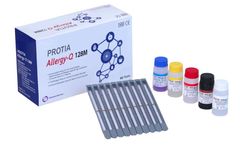
PROTIA Allergy-Q 128M panel is an in vitro diagnostic test in the quantitative determination of allergen-specific IgE concentrations in human serum or plasma using immunoblotting technique. Diagnose 118 types of allergens including Total IgE in one ...
Manufactured by:ORGENTEC Diagnostika GmbH is part of the Sebia Group based inMainz, GERMANY
This assay contributes to the diagnosis of antiphospholipid syndrome (APS). Regardless of their immunoglobulin class, cardiolipin autoantibodies are an important criterion for the diagnosis of APS. Autoantibodies against cardiolipin are also an immunological classification criterion for systemic lupus erythematosus (SLE). In addition, the quantitative determination of anti-cardiolipin is very ...
Manufactured by:PROTIA. iNC, formely known as ProteomeTech Inc. based inSeoul, SOUTH KOREA
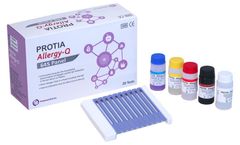
PROTIA Allergy-Q 64S panel is an in vitro diagnostic test in the quantitative determination of allergen-specific IgE concentrations in human serum or plasma using immunoblotting technique. Diagnose 63 types of allergens including Total IgE in one ...
Manufactured by:Sansure Biotech Inc. based inChangsha, CHINA
15 High-risk HPV DNA Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the detection of high-risk HPV (type 16, 18, 31, 33,35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68) present in exfoliated cells from females’ cervix. The test results can be used as an aid in the diagnosis of a high-risk HPV infection. ...
Manufactured by:NanoEnTek, Inc. based inGuro-gu, SOUTH KOREA
BUDDI™ is an image analyzer that evaluates the line signal intensities on the test ...
Manufactured by:Nemera based inLa Verpillière, FRANCE
Quality and performance for ENT sprays in regulated markets. The SP270+ and SP370+ are our versatile pumps for both Rx/ OTC drug products and medical devices for ear, nose and throat sprays. They comply with regulated markets requirements. ...
Manufactured by:Sansure Biotech Inc. based inChangsha, CHINA
This High-risk HPV DNA (Genotype) Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the detection of high-risk HPV (type 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68) present in exfoliated cells from females’ cervix. The test results can be used for identification of HPV ...
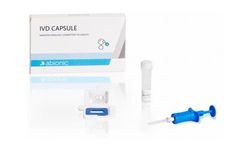
Manufactured by:Abionic SA based inEpalinges, SWITZERLAND
The IVD CAPSULE Aeroallergens is the first rapid, single-use in vitro diagnostic test for the quantitative determination of circulating immunoglobulin E (IgE) specific to the allergen components Phl p 1 + Phl p 5 (combined), Der p 1 + Der p 2 (combined), Alt a 1, Fel d 1 and Can f 1 in uncoagulated human capillary blood collected from the patient’s ...
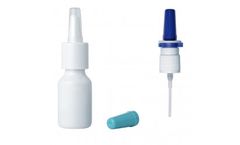
Manufactured by:Nemera based inLa Verpillière, FRANCE
Off-the-shelf devices available for key identified nasal drugs. Accelerate your sprint to market with our Device Equivalence Program. Based on FDA requirements, we have established a Device Equivalence Program in order to accelerate time-to-market and achieve in-vitro bioequivalence requirements. Our main objective is to preselect and propose an appropriate delivery system per identified drug ...