Refine by
Medical Device Prototype Development Equipment & Supplies
60 equipment items found
by:Sterling Medical Devices based inMoonachie, NEW YORK (USA)
Prototyping is a learning process and trials must be done diligently with attention to detail to avoid extraneous costs that might hinder the product from reaching market mass production. During this time, hundreds of exact replicas may be needed to verify and validate a design. Without proper management, redesigns can become a burden with each respective batch that needs to be reworked. Sterling ...
Manufactured by:Novara Technologies Ltd based inHartley Wintney, UNITED KINGDOM
Medical device companies are actively developing an increasing interest in electronics for disposable devices. Disposable devices are designed to be sterilised and used only one time and as a result unit cost can often be reduced by using a wider choice of engineering ...
Manufactured by:Teleflex Medical OEM based inPlymouth, MINNESOTA (USA)
Teleflex Medical OEM specializes in providing mandrel wire coated with their proprietary PD-Slick™ material, which is a composite of polyimide and PTFE. This coating acts as a processing aid in the manufacturing of high-precision tubing internal diameters. Serving as a comprehensive solution provider, Teleflex Medical OEM offers a vertically-integrated ...
by:Fizimed SAS based inStrasbourg, FRANCE
The Emy biofeedback Kegel trainer is an innovative and patented medical device developed in France. Fizimed believes in responsible production. Therefore, all our parts come from France, Germany, and Portugal. Furthermore, we verify the origin and quality of our materials to ensure our products are perfectly safe to use. Emy allows women of all ages to strengthen their pelvic floor alone from the ...
Manufactured by:Nextern Inc based inWhite Bear Lake, MINNESOTA (USA)
Nextern is a collaborative medical device company. We bring an unmatched core competence in medical device product design and development through manufacturing as a leverageable asset to collaborators like you, who are focused on accelerating the commercial side of their ...
Manufactured by:Mechatronic GmbH based inDarmstadt, GERMANY
Medical devices are used in network environments more and more often these days. There are numerous applications, among which data transfer in the context of device monitoring, preventive maintenance and update-capacity are the most common. Cyber security is of special importance when it comes to medical and diagnostic devices. After all, there are human lives at stake.Malware must be ...
Manufactured by:Pierrel S.p.A., based inCapua (CE), ITALY
Goccles is a medical device made in Italy developed by the Catholic University of Rome, that uses the common curing light present in every dental office to perform a quick and non-invasive examination of the autofluorescence of the oral cavity, identifying any precancerous and cancerous ...
Manufactured by:Optimum Medical based inLeeds, UNITED KINGDOM
OptiLube sterile lubricating jelly is a medical device, developed to aid the smooth insertion of medical devices during a range of examinations and procedures. OptiLube is water-soluble, transparent, and formulated to adhere well to gloves and instruments. The use of sterile lubricating jelly during procedures and examinations reduces the risk of pain and trauma to the patient, by reducing ...
Manufactured by:AgPlus Diagnostics Ltd based inSharnbrook, UNITED KINGDOM
To work in conjunction with our proven sensitive assay development service, we are proud to have launched The Agilis Reader. This pairing delivers the market requirement of a rapid, ultra-sensitive, fully quantitative Point of Care test for use across a wide range of clinical and non-clinical applications. The multi-application capability of the new AgPlus platform will see it meeting many ...
by:MPR Associates based inAlexandria, VIRGINIA (USA)
MPRs electrical and software engineers work on a wide range of technologies to develop embedded systems for medical devices and other products. We employ a number of platform accelerators preconfigured hardware integrated with software to reduce time-to-market with proven technologies. Our expertise covers wearable devices, portable battery backed systems, and large scale systems. Our software ...
Manufactured by:MPE Inc. based inMilwaukee, WISCONSIN (USA)
The Robotic Navigation Development Kit offered by MPE Inc. is specifically designed to expedite the market entry of new OEM medical devices by integrating with the KUKA Development Kit. This solution is targeted at start-up and scale-up OEMs aiming to significantly cut down the development time required for their medical devices. The kit allows for seamless integration of robotic navigation ...
by:Sterling Medical Devices based inMoonachie, NEW YORK (USA)
In the general perspective, product design necessitates that your product can fundamentally work for its purpose, be aesthetically well received by its users, and be economically manufactured with cost-effective materials. However, the high-quality demands of industry markets can become a challenge when approached independently. Sterling’s medical device industrial design consultations ...
by:Sterling Medical Devices based inMoonachie, NEW YORK (USA)
Medical product design is a crucial phase in the product development lifecycle. A mix of art and science, the design of life-improving medical devices that align with healthcare regulatory requirements and meet end-user needs is a complex process that requires innovation, precision, and advanced ...
Manufactured by:Neofect based inWatertown, MASSACHUSETTS (USA)
Importance of Functional Arm Reaching Training. The Smart Board is a virtual-reality adapted mobile arm support, effectively improving range of motion and coordination of the shoulder and elbow joints. Smart Board for Upper Extremity Exercise following Stroke. Neofect Smart Board is a high-tech medical device that develops users' shoulder and upper arm movements to promote improved functional ...
Manufactured by:MedSpark, LLC based inSan Luis Obispo, CALIFORNIA (USA)
During Total Knee Arthroplasty (replacement) or TKA, a patellar component (commonly composed of UHMW polyethylene) is typically located by the surgeon on the posterior aspect of the patient's existing patella. The patellar surface must be prepared by the surgeon to accept the patellar component and a patella clamp instrument is typically utilized by surgeons during this ...
Manufactured by:MEDITULIP Co., Ltd. based inChungcheongbuk-do, SOUTH KOREA
MEDITULIP developed a novel asymmetrical linear stapler (NALS) that enables surgeons to take tissue samples for frozen section biopsy from the true resection margin and to confirm the radicality of cancer surgery intraoperatively. MEDITULIP patented this technology and develops other medical devices to advance minimally invasive surgery. MEDITULIP is preparing for regulatory approval of its ...
by:BICO - The Bio Convergence Company based inBoston, MASSACHUSETTS (USA)
Enabling high-throughput 3D cell culturing and bioprinting to create the future of health. The epitome of user-friendly flexibility. Bioprint with more materials for even more complex models. Independent dual pressure that enables coaxial and mixing printing. Create multimaterial constructs with a mix of crosslinking modalities thanks to six printheads. 6 printheads enabling more complex ...
Manufactured by:Eliaz Therapeutics based inSanta Rosa, CALIFORNIA (USA)
Acute Kidney Injury (AKI) and sepsis are major public health concerns, with high incidence, mortality and morbidity. Despite efforts to develop new interventions, current therapeutic options are limited in scope and effectiveness. At Eliaz Therapeutics, we have developed a patented, unique medical device that will remove Galectin-3 (Gal-3) from human blood. Our device removes both bound and free ...
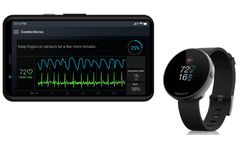
Manufactured by:CardiacSense based inCaesarea, ISRAEL
Performance You Can Count On: The CardiacSense watch is the most advanced, clinically proven solution in cardiac monitoring. Reliable as it is innovative, it is the novel way to ensure constant monitoring of the cardiac system and blood pressure, 24 hours a day, 365 days a year, for as long as it’s ...
Manufactured by:eg Technology Ltd based inStow-cum-Quy, UNITED KINGDOM
IMOD is the only responsive oxygen delivery device able to monitor and automatically adjust to a patient’s individual oxygen needs and requirements. The device receives oxygen from a bottled or bulk supply and reactively delivers the correct dose through a nasal cannula or a face mask, the first in its class to do so. The ultra-sensitive breath-detection system at the heart of IMODtracks ...