- Home
- Equipment
- usa california
- oncology
Refine by
Applications
- SeeFactor CT3 for Consider Ear, Nose & Throat (ENT)
- SeeFactor CT3 for Consider Orthopedics (Hips, Knees, Ankles, Bone Density)
- SeeFactor CT3 for Consider Cranial and Spine
- SeeFactor CT3 for Consider Lung Imaging
- Intraoperative Radiation Therapy (IORT) System - Electron Therapy for Skin Cancer
- Intraoperative Radiation Therapy (IORT) System for Flash Radiotherapy
- Intraoperative Radiation Therapy (IORT) System for Pancreatic Cancer Treatment
- Intraoperative Radiation Therapy (IORT) System for Breast Cancer Treatment
- Intraoperative Radiation Therapy (IORT) System for Single-Fraction APBI
- Intraoperative Radiation Therapy (IORT) System for Surgical Oncologists
- Intraoperative Radiation Therapy (IORT) System for Radiation Oncologists
- Intraoperative Radiation Therapy (IORT) System for Hospital Administrators
- Remote Patient Monitoring Solution for Remote Patient Monitoring
- Remote Patient Monitoring Solution for Revenue Cycle Management
- Remote Patient Monitoring Solution for EHR Integration
Oncology Equipment Supplied In Usa California
65 equipment items found
Manufactured by:XILIS, Inc. based inDurham, NORTH CAROLINA (USA)
Xilis’ MicroOrganoSphereTM powers the only assay which can provide oncologists with accurate results of drug sensitivity on a patient’s own tumor within the clinical decision-making window - finally realizing the promise of precision medicine. Xilis is developing a panel of disease specific assays systematically testing the most important standard-of-care drugs within equipoise or ...
Manufactured by:Ibio, Inc. based inBryan, TEXAS (USA)
Advances in the field of immuno-oncology have led to new and better treatment outcomes for a range of cancers, and particularly, blood cancers. However, even with the advent of checkpoint inhibitors as immunotherapies for solid tumors, significant challenges remain. This is in part due to dynamics in the tumor microenvironment, wherein regulatory T cells [Tregs] proliferate and ...
Manufactured by:Aelan Cell Technologies based inSan Francisco, CALIFORNIA (USA)
Cancer survival rates decrease with age, in part because the efficacy of cancer therapies decline for older ...
Manufactured by:Fate Therapeutics, Inc. based inSan Diego, CALIFORNIA (USA)
The oncology therapeutic landscape has been transformed with the use of checkpoint inhibitor therapy. Despite the clinical benefit conferred by approved checkpoint inhibitor therapy against a variety of tumor types, these therapies are not curative and, in most cases, patients either fail to respond or their disease progresses on these agents. ...
Manufactured by:Senti Biosciences based inSouth San Francisco, CALIFORNIA (USA)
Senti Bio believes there are several advantages that NK cells confer in relation to other potential immune cell types in oncology: innate killing, immune activation, previously validated efficacy and safety, and broad patient access since they may be delivered rapidly to patients in an off-the-shelf manner and in outpatient settings. ...
Manufactured by:Lineage Cell Therapeutics, Inc. based inCarlsbad, CALIFORNIA (USA)
VAC2 is an allogeneic, or non-patient specific, cancer vaccine candidate designed to stimulate patient immune responses to telomerase which is commonly expressed in cancerous cells but not in normal adult cells. VAC2, which is produced from our pluripotent cell technology using a directed differentiation method, is comprised of a population of mature dendritic cells. As the most potent type ...
by:Natera, Inc. based inAustin, TEXAS (USA)
Support treatment decisions with comprehensive tumor profiling. Gain deep insight into the tumor’s genomic alterations and oncology biomarkers with Altera. The detailed sequencing report includes FDA-approved treatments, novel treatment approaches and ongoing clinical trials targeting found mutations. Unlike any other tumor profiling assay, Altera also enables molecular ...
Manufactured by:BridgeBio Pharma, Inc. based inPalo Alto, CALIFORNIA (USA)
In 2022, Helsinn Group gained full, exclusive licensing rights to commercialize high-dose infigratinib worldwide in oncology indications except in mainland China, Hong Kong and Macau. BridgeBio has established a strategic collaboration with LianBio for the development and commercialization of infigratinib in oncology indications in mainland China, Hong Kong and ...
Manufactured by:BridgeBio Pharma, Inc. based inPalo Alto, CALIFORNIA (USA)
RAS is one of the most well-known oncogenic drivers, with approximately 30% of all cancers being driven by RAS mutations, including large proportions of lung, colorectal and pancreatic tumors. BridgeBio’s approach to RAS cancers encompasses multiple “shots on goal” that target both KRAS mutant tumors and those in which RAS activates PI3Ka. We believe two of our approaches have ...
Manufactured by:BridgeBio Pharma, Inc. based inPalo Alto, CALIFORNIA (USA)
Recent high profile publications provide preclinical in vivo rationale for monotherapy and combinations with immuno-oncology agents, kinase inhibitors, and chemotherapy. Optimization of oral lead compounds is ...
Manufactured by:BridgeBio Pharma, Inc. based inPalo Alto, CALIFORNIA (USA)
BridgeBio is developing SHP2 inhibitors as potentially effective additions to the therapeutic arsenal for difficult-to-treat cancers. SHP2, encoded by the PTPN11 gene, links growth factor signaling with the downstream RAS/ERK/MAPK pathway to regulate cell growth and division. Over-activity of this pathway, often driven by distinct gene mutations, causes or contributes to many human cancers. ...
by:Natera, Inc. based inAustin, TEXAS (USA)
Signatera is a personalized, tumor-informed assay optimized to detect circulating tumor DNA (ctDNA) for molecular residual disease (MRD) assessment and recurrence monitoring for patients previously diagnosed with cancer, with broad utility for cancer ...
Manufactured by:ICU Medical, Inc. based inSan Clemente, CALIFORNIA (USA)
Maximize efficiency and safety by remotely verifying every mix without having to gown up and enter the cleanroom. Diana is the first and only system to combine both automated compounding and IV workflow technologies with wireless pharmacist notification to let you remotely verify each preparation on a handheld tablet display. Integrated high-definition cameras let you visualize the workflow for ...
Manufactured by:Gilead based inFoster City, CALIFORNIA (USA)
Sacituzumab govitecan-hziy 180 mg for injection. ...
Manufactured by:Gilead based inFoster City, CALIFORNIA (USA)
Brexucabtagene autoleucel . TECARTUS.TECARTUS®(brexucabtagene autoleucel)suspension for intravenous infusion. ...
Manufactured by:ICU Medical, Inc. based inSan Clemente, CALIFORNIA (USA)
With the lowest cost to implement, ChemoClave makes the decision to start improving IV medication safety a whole lot easier. We know that the cost of implementing a CSTD into clinical practice can be a deciding factor in the purchasing process. That’s why we developed the ChemoClave needlefree CSTD to not only help you minimize hazardous drug exposure and maximize medication safety, but to ...
Manufactured by:ICU Medical, Inc. based inSan Clemente, CALIFORNIA (USA)
Treating cancer patients takes compassion—it shouldn’t take a toll on your health. But the fact is, while chemotherapy is the backbone of medical oncology, these powerful medications—along with many others—are extremely toxic to the clinicians who handle them. Prolonged exposure to hazardous drug vapors, drips, or spills has been shown to lead to hair ...
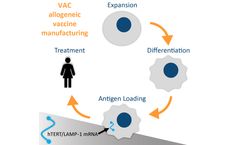
Manufactured by:Sana Biotechnology based inSeattle, WASHINGTON (USA)
Oncology - Multiple ...
Manufactured by:Tempus based inChicago, ILLINOIS (USA)
Targeted panel designed to detect actionable oncological targets in plasma. ...
Manufactured by:Sana Biotechnology based inSeattle, WASHINGTON (USA)
Oncology: Multiple ...