Refine by
Patient Access Equipment Supplied In Belgium
7 equipment items found
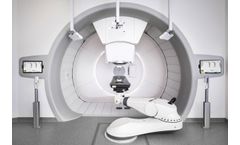
by:IBA based inLouvain-La-Neuve, BELGIUM
It is a compact technology that is easy to install, integrate, operate and finance. It is also scalable, so you can expand over time. Proteus®ONE focuses on patient comfort and accessibility while delivering the most clinically advanced form of proton radiation therapy. Thanks to pencil beam scanning, it minimizes radiation exposure to healthy tissue. It also ...
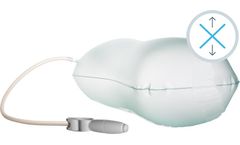
Manufactured by:Ergotrics NV based inTurnhout, BELGIUM
Two shapes, two sizes, three versions: a dozen different variations. Proper positioning of the patient provides optimal access to the surgical or medical procedure site while maintaining body function and structural integrity. Using compressed air instead of manual labor is an ergonomic breakthrough in the hospital. Thanks to the different shapes and variations, ...
Manufactured by:IdenPro BV based inHaacht, BELGIUM
IdenPro’s i-Band series RFID direct thermal printable wristbands are a crucial part of patient admission system and helps improve its workflow efficiency by connecting patients and the hospitals IT infrastructure. It also helps aid patient ...
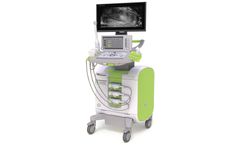
Manufactured by:Exact Imaging based inMarkham, ONTARIO (CANADA)
ExactVu micro-ultrasound system for targeted biopsies for prostate cancer. ...
Manufactured by:Axinesis based inWavre, BELGIUM
Deliver neurorehabilitation at home with REAtouch® Lite by Axinesis. The REAtouch® Lite portable rehabilitation device enables intensive functional neurorehabilitation in settings ranging from a rehabilitation centre to the patient’s ...
Manufactured by:OraSure Technologies based inBethlehem, PENNSYLVANIA (USA)
The OraQuick® Ebola Rapid Antigen Test has been granted De Novo 510(k) clearance by the U.S. Department of Health and Human Services for the following intended use: The OraQuick® Ebola Rapid Antigen Test is an in vitro diagnostic single-use immunoassay for the qualitative detection of antigens from viruses within the Ebolavirus genus but does not differentiate between these viruses. ...
Manufactured by:OraSure Technologies based inBethlehem, PENNSYLVANIA (USA)
The OraQuick® Ebola Rapid Antigen Test has been granted De Novo 510(k) clearance by the U.S. Department of Health and Human Services for the following intended use: The OraQuick® Ebola Rapid Antigen Test is an in vitro diagnostic single-use immunoassay for the qualitative detection of antigens from viruses within the Ebolavirus genus but does not differentiate between these viruses. ...