Refine by
Applications
- Drug Dolution for Liver Metastases
- Drug Dolution for Gastric Cancer
- Microbiota for Restoration Therapy
- Microbiota Based Product for Physician-Sponsored Studies
- Microbiome Therapeutics for Patients and Physicians
- Remote Patient Monitoring Solution for Remote Patient Monitoring
- Remote Patient Monitoring Solution for Revenue Cycle Management
- Remote Patient Monitoring Solution for EHR Integration
Patient Enrollment Equipment & Supplies
49 equipment items found
Manufactured by:Biocartis NV based inMechelen, BELGIUM
IdyllaTM ctKRAS covers 21 KRAS mutations in exons 2,3 and 4 and showed an high concordance to NGS of 96% using plasma samples from 198 mCRC patients enrolled in the prospective multicenter RASANC ...
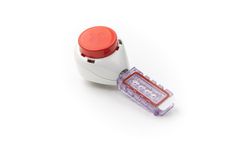
Manufactured by:Tasso, Inc. based inSeattle, WASHINGTON (USA)
The Tasso-M20 device delivers whole dried blood samples from the patient to the lab. It can be used for PK (pharmacokinetic) monitoring in patients enrolled in clinical trials. Subjects can successfully collect volumetrically precise samples regardless of access to trial sites. The Tasso-M20 is CE marked and FDA ...
Manufactured by:Veracyte, Inc. based inSouth San Francisco, CALIFORNIA (USA)
LymphMark (Lymphoma) – We are developing this lymphoma subtyping test as a companion diagnostic for Acerta Pharma and AstraZeneca’s acalabrutinib (Calquence®). In April 2021, we announced that the first patient has been enrolled and randomized in Acerta Pharma’s Phase 3 ESCALADE trial, which uses the investigational LymphMark test to ...
Manufactured by:Longenesis based inRiga, LATVIA
Collect Real World Data and patient outcomes; Longenesis.Engage is a digital patient engagement platform that enables the benefits of digital approach to patient engagement and research execution. Conduct multiple research projects, funnel patient cohorts for upcoming studies, follow-up and re-engage the participants, and manage their consent while upholding the data protection regulations and ...
Manufactured by:CytomX Therapeutics, Inc. based inSouth San Francisco, CALIFORNIA (USA)
Bristol Myers Squibb is enrolling patients in the dose escalation phase of a Phase 1/2a clinical trial (NCT03994601) of a second anti-CTLA-4 Probody, BMS-986288, based on a modified version of Yervoy® (ipilimumab), to evaluate a CTLA-4-directed Probody therapeutic alone or in combination with Opdivo® (nivolumab) in patients with selected ...
by:Abivax based inParis, FRANCE
ABX464 is an oral, first-in-class, small molecule that has demonstrated safety and profound anti-inflammatory activity in the completed Phase 2a and Phase 2b induction studies as well as in ongoing long-term maintenance studies in patients with moderate-to-severe ulcerative colitis (UC).Due to the clinical similarities of Crohn’s disease (CD) and UC, and the predictability of the DSS model ...
Manufactured by:atHeart Medical based inBaar, SWITZERLAND
Defects vary in size. Some may close on their own early in life, while others require an intervention. Today, the vast majority are performed through minimally invasive catheter-based interventions. They consist in implanting an atrial septal occluder through a transcatheter procedure to close the defect. Current ASD occluder devices have dense metal frames that permanently clamp the septum. The ...
Manufactured by:SystImmune Inc. based inRedmond, WASHINGTON (USA)
Built on the foundation of Specificity Enhanced Bi-specific Antibody (SEBA) molecules the SEBA-ADC have an advanced mechanism of action. This advanced functional class is referred to as Heterogeneity overcoming - Immunogenic death inducing - Resistance antagonizing - Enhanced Specificity (HIRE) molecules that target cancer cells. While HIRE molecules provide cancer cells specific targeting, and ...
by:Stemline Therapeutics, Inc. based inNew York, NEW YORK (USA)
SL-801’s potential ability to reversibly bind XPO1 may offer the possibility to mitigate side effects and help optimize the therapeutic index. A multicenter Phase 1 dose escalation trial of single agent SL-801 is currently enrolling patients with advanced solid ...
Manufactured by:AOBiome Therapeutic, LLC based inCambridge, MASSACHUSETTS (USA)
We have completed six Phase 2 clinical programs and multiple pre-clinical programs. Over 1,000 patients have been enrolled in our clinical trials since 2016. All of those patients except for 28 in our open label pediatric study have been enrolled in randomized double-blind placebo controlled clinical trials evaluating both ...
Manufactured by:Sense Neuro Diagnostics based inCincinnati, OHIO (USA)
Sense is developing non-invasive brain scanners that enable faster detection and triage, and continuous monitoring of brain injury, to improve patient ...
by:Castle Biosciences, Inc. based inFriendswood, TEXAS (USA)
Our DecisionDx-CMSeq is a 3-gene test that uses next-generation sequencing (NGS) to identify somatic mutations relevant to cutaneous melanoma in melanoma tumor tissue. The test includes hotspot mutations in the genes BRAF, NRAS and ...
Manufactured by:SystImmune Inc. based inRedmond, WASHINGTON (USA)
The SI-B001 primary mechanism of action is the blocking of EGFR and HER3 signals to cancer cells, and secondarily, through a wt FC receptor mediating innate immune effector functions toward the cancer cells. SI-B001 is currently being evaluated as a single agent in clinical trials enrolling patients with a variety of solid tumor ...
Manufactured by:Nkarta, Inc. based inSouth San Francisco, CALIFORNIA (USA)
OX40 costimulatory domain, CD3ζ signaling moiety, membrane bound ...
Manufactured by:SystImmune Inc. based inRedmond, WASHINGTON (USA)
The specificity enhancement both synergistically enhance and expand the breadth of immune cell activity that is diminished in cancer patients (SEBA). The spatio-temporal control of bi-specificity ensure that synergistic blocking of inhibitory receptors is happening in the right place for greatest effect. The tetravalent SI-B003 has two binding domains for T cell checkpoint axis, ...
Manufactured by:Rebiotix Inc., a Ferring Company based inRoseville, MINNESOTA (USA)
Rebiotix Inc., a Ferring Company, has conducted several trials of the lead microbiota-based product in the MRT drug platform, RBX2660, for reducing the rate of recurrence of Clostridioides difficile infection. RBX2660 is currently under study in a Phase 3 clinical trial in conjunction with the US Food and Drug Administration’s Investigational New Drug ...
Manufactured by:Seres Therapeutics, Inc. based inCambridge, MASSACHUSETTS (USA)
SER-109 is a first-in-class investigational microbiome therapeutic administered orally following antibiotics to reduce recurrence of C. difficile infection (CDI) in patients with recurrent ...
Manufactured by:SystImmune Inc. based inRedmond, WASHINGTON (USA)
Antibody Drug Conjugates (ADC) molecules utilize specific antibodies to deliver therapeutic small molecules specifically to cancer cells. ADC are created in cooperation with Bai-Li Pharmaceutical’s R&D Center of Excellence and enable the development of sophisticated chemistries that give this class of drugs their therapeutic payload. This advanced functional class is referred to ...
Manufactured by:SystImmune Inc. based inRedmond, WASHINGTON (USA)
Antibody Drug Conjugates (ADC) molecules utilize specific antibodies to deliver therapeutic small molecules specifically to cancer cells. ADC are created in cooperation with Bai-Li Pharmaceutical’s R&D Center of Excellence and enable the development of sophisticated chemistries that give this class of drugs their therapeutic payload. This advanced functional class is referred to ...
Manufactured by:Galapagos NV based inMechelen, BELGIUM
Filgotinib, a preferential JAK1 inhibitor, is marketed as Jyseleca in the European Union (incl. Norway), Great Britain, and Japan for the treatment of adults with moderately to severely active rheumatoid arthritis (RA) who have responded inadequately or are intolerant to one or more disease modifying anti-rheumatic drugs ...