Show results for
Refine by
Patient Program Management Equipment Supplied In Canada
6 equipment items found
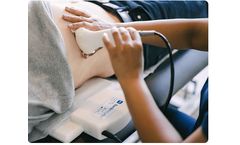
Manufactured by:Sonic Incytes Medical Corp. based inVancouver, BRITISH COLUMBIA (CANADA)
Velacur is a portable, handheld ultrasound that integrates a live ultrasound image, resulting in an assessment of liver stiffness and attenuation, components of liver fibrosis and fat measurements. It generates real-time stiffness measurements at point of care with technology similar to that of MRI elastography. Velacur is safe and effective as an aid in the management of patients with liver ...
Manufactured by:Beckman Coulter, Inc. based inBrea, CALIFORNIA (USA)
The iRICELL series combines urine chemistry and microscopy in a fully automated, walk-away workcell that is easy to use and maintain. Optimize and advance urinalysis and body fluid testing in your laboratory with proprietary Digital Flow Morphology technology that uses Auto-particle Recognition (APR) software to deliver standardized and accurate ...
Manufactured by:Response Biomedical Corp. based inVancouver, BRITISH COLUMBIA (CANADA)
Procalcitonin (PCT) is a biomarker elevated in the blood of patients suffering from sepsis. Physicians use PCT to distinguish bacterial sepsis from other causes of similar symptoms. RAMP Procalcitonin provides quantitative results in ~15 minutes directly from a small blood sample. When speed, precision and accuracy are critical, the RAMP Procalcitonin test provides clinicians the results they ...
Manufactured by:Beckman Coulter, Inc. based inBrea, CALIFORNIA (USA)
The iQ200 series automated urine microscopy analyzer produces shortened TAT with standardized results. By leveraging proprietary Digital Flow Morphology technology using Auto-Particle Recognition (APR) Software, urine particles are isolated, identified and characterized on the screen to virtually eliminate the need for manual microscopy. The iQ200 series analyzers are available as either ...
Manufactured by:Response Biomedical Corp. based inVancouver, BRITISH COLUMBIA (CANADA)
Response Biomedical offers a FDA cleared whole blood immunoassay NT-proBNP test, with our proprietary RAMP technology which detects the levels of N-terminal prohormone of brain natriuretic peptide in patients with congestive heart failure around the world. Read more about how this product will help your physicians and patients where a traditional BNP test ...
Manufactured by:Augurex Life Sciences Corp. based inVancouver, BRITISH COLUMBIA (CANADA)
Extracellular 14-3-3η protein is a potent ligand and activator of intracellular signalling pathways that lead to the up-regulation of inflammation and joint damage factors involved in RA pathogenesis. The 14-3-3η blood test is clinically available as a diagnostic test whereby a positive result indicates a 5 to 50 times greater likelihood of having RA and informs joint damage prognosis and ...