- Home
- Equipment
- north america
- pediatric disease
Show results for
Refine by
Pediatric Disease Equipment Supplied In North America
9 equipment items found
by:Aegle Therapeutics based inWoburn, MASSACHUSETTS (USA)
Epidermolysis bullosa (“EB”) is a group of rare genetic disorders that manifests as blistering or erosion of the skin in response to little or no apparent trauma. There are many genetic and symptomatic variations of EB. EB is always painful, often pervasive and debilitating, and is in some cases lethal before the age of 30. EB affects both genders and every racial and ethnic ...
Manufactured by:HemoShear Therapeutics, Inc. based inCharlottesville, VIRGINIA (USA)
HST5040 is formulated for convenient daily administration at home as a liquid taken either orally or through a gastrostomy tube. The FDA has granted HST5040 Orphan Drug, Fast Track and Rare Pediatric Disease designations for the treatment of MMA and ...
Manufactured by:Diagnosys LLC based inLowell, MASSACHUSETTS (USA)
Ideal for Pediatric, Neurology, and Operating Room testing. Features: Advanced capabilities, ColorFlash has a flash luminance from 0.01 to 1,000 cd·s/m2, Any flash or background color: red, green, blue & white LEDs, Test patients at 30 cm (~1 ft), or any distance from 0 to 3 meters, ...
Manufactured by:Diagnosys LLC based inLowell, MASSACHUSETTS (USA)
Ideal for Pediatric and Operating Room testing. Features: Advanced flash capabilities, ColorBurst™ has a >10 billion-to-1 luminance range, Flash durations of nanoseconds to minutes, Any color background from red, green & blue LEDs, Easy to use, ultimate performance: Auto calibration, Buttons on handle control testing, User programmable arbitrary waveforms with 1 ms, resolution ...
Manufactured by:Merete Technologies, Inc. (MTI) based inOakbrook Terrace, ILLINOIS (USA)
To ensure that the staples will behave rigidly and mechanically, their central areas are reinforced especially well, allowing stable, precise temporary epiphysiodesis. The trapezoidal design of the PediatrOS™ RigidTack™ staple is closely aligned to the anatomy of the femur and tibia. Cannulated legs allow accurate placement using K-wires. Low fluoroscopy times are another advantage. ...
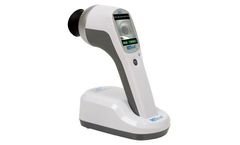
Manufactured by:Konan Medical USA, Inc. based inIrvine, CALIFORNIA (USA)
RETeval is the only FDA 510(k) cleared, non-mydriatic* full-field ERG device that is easier to use than you might think. Many diseases and disorders affect various cell populations in the retina, and ERG testing provides an objective, non-invasive method of evaluating retinal function. Hand-held, battery-powered & easy to use anywhere, all day, with easy interpretation aided by a 500+ ...
Manufactured by:BiomX based inNess Ziona, ISRAEL
We are developing BX005, a topical phage cocktail that targets Staphylococcus aureus (S. aureus), a bacteria associated with the manifestation of the disease. This program leverages know-how and capabilities acquired under our previous programs with regard to topical formulation and clinical testing. In preclinical in vitro studies, BX005 was shown to be active against over 90% of strains of S. ...
Manufactured by:Merete Technologies, Inc. (MTI) based inOakbrook Terrace, ILLINOIS (USA)
For correcting axis misalignment (Genu varum/valgum) using hemi-epiphysiodesis. The flexible center area of the anatomically shaped PediatrOS™ FlexTack™ allows it to bend open in vivo in response to bone growth forces, helping steer pediatric and adolescent bone growth gently and precisely. The trapezoidal design of the PediatrOS™ FlexTack™ staple is closely aligned to the ...
Manufactured by:Virpax Pharmaceuticals based inBerwyn, PENNSYLVANIA (USA)
Benefits: NobrXiol may be using significantly less pharmaceutical-grade CBD than current FDA approved oral CBD dosing. NobrXiol may achieve higher efficiency via the nasal route and reduce peripheral side effects like dose dumping due to high-fat meals. Since peripheral exposure via the plasma will be reduced by the nose to brain delivery, we believe there will be negligible liver first-pass ...