Refine by
Pre Clinical Effectiveness Case Study Equipment & Supplies In Ethiopia
166 equipment items found
Manufactured by:Tectonic Therapeutic, Inc. based in
AVROBIO has a preclinical research program for an investigational gene therapy for Gaucher disease type ...
Manufactured by:Tectonic Therapeutic, Inc. based in
AVROBIO has a preclinical research program for a gene therapy for Pompe disease. We have shown a favorable preclinical safety and efficacy profile in a mouse model of Pompe ...
Manufactured by:Tectonic Therapeutic, Inc. based in
AVROBIO has a preclinical research program for mucopolysaccharidosis type II (MPS II), or Hunter syndrome. The program is being developed in collaboration with Brian Bigger, Ph.D., who has published favorable preclinical data in a mouse model of Hunter ...
Manufactured by:CTIBiotech based inMeyzieu, FRANCE
Using the latest tools in bioengeneering, our researchers are now printing cancer tissues in 3D. These models can be used for highly significant pre-clinical trials and can be produced in 6/48/96 well plates for high throughput ...
Manufactured by:Elpiscience based inShanghai, CHINA
ES004 is a potentially best-in-class antibody targeting pan-allele signal-regulatory protein alpha (SIRPα). SIRPα is an inhibitory receptor expressed only on myeloid cells and dendritic cells. Binding of CD47 to SIRPα delivers a “don’t eat me” signal to suppress phagocytosis. Tumor cells frequently overexpress CD47 to evade macrophage-mediated destruction. To ...
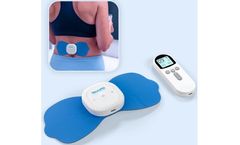
Manufactured by:NeuroMD based inNorth Miami Beach, FLORIDA (USA)
The most advanced, FDA-cleared system to restore your lower back health and provide lasting pain relief. Equipped with pre-set, clinically studied NMES parameters, the Corrective Therapy Device® delivers proven electrical stimulation to correct the source of the pain. Get started on the path to relief during our biggest sale ever with savings of $200 and enjoy a 60-day at-home ...
Manufactured by:Elpiscience based inShanghai, CHINA
LAG3 is an immune checkpoint receptor known to suppress T cell activation. We designed ES005 to block LAG3 ligands, including MHC class II and FGL1. Through unique epitope binding, ES005 is able to recognize human LAG3 with high affinity. In preclinical studies, ES005 inhibited tumor growth both as a monotherapy and in combination with a PD-1 ...
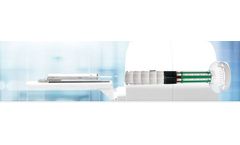
Manufactured by:MR Solutions based inGuildford, UNITED KINGDOM
The MRS*PET INSERT, designed by MR Solutions, facilitates simultaneous PET/MR imaging, enabling the acquisition of multi-parametric information from the same subject with precision. Built to integrate seamlessly with cryogen-free MR systems up to 9.4 Tesla, it offers flexibility in research settings due to its removable nature. This product can function independently of the MR system, effectively ...
Manufactured by:Mirus Bio LLC based inMadison, WISCONSIN (USA)
TransIT-VirusGEN SELECT Transfection Reagent is designed to enhance delivery of packaging and transfer vector DNA to suspension and adherent HEK 293 cell types in order to increase production of recombinant lentivirus and adeno-associated virus (AAV). Key benefits of TransIT-VirusGEN SELECT Transfection Reagent include: Performance – Efficient DNA delivery for large-scale production of ...
Manufactured by:Wolfmet based inManchester, UNITED KINGDOM
Wolfmet 3D, developed by M&I Materials Ltd, leverages a sophisticated selective laser melting (SLM) technique for additive manufacturing. This process involves the use of a high-powered laser to meticulously fuse layers of pure tungsten powder. The result is the creation of highly intricate and precise components such as collimators and radiation shields used in medical imaging systems like ...
by:Verb Biotics based inBoston, MASSACHUSETTS (USA)
Yso2 is a preclinical program targeting Crohn’s disease. It aims to develop Christensenella gut bacteria as a safe, efficient and groundbreaking oral biotherapy. YSOPIA has identified a lead candidate to relieve Crohn’s disease associated symptoms and slow down disease progression. Discover the articles we published in Scientific Reports: Relizani et al. 2022 and Kropp et al. 2021. ...
Manufactured by:AstraZeneca based inCambridge, UNITED KINGDOM
IVX-121 targets respiratory syncytial virus (RSV), a major cause of viral pneumonia for which no vaccine has been FDA approved. IVX-121 incorporates a stabilized prefusion F antigen licensed from NIAID/NIH (DS-Cav1; Science 2019). RSV F is known to undergo major structural changes that allow viral entry into the host cell, and during that process, critical protective epitopes are lost. Protein ...
Manufactured by:Biopeptek Pharmaceuticals LLC based inMalvern, PENNSYLVANIA (USA)
Biopeptek is a leading manufacturer of synthetic peptide Active Pharmaceutical Ingredient (API). We offer custom APIs for pharmaceutical research & development organizations world-wide to support pre-clinical and clinical studies. We also support our clients for preparation of Chemistry, Manufacturing and Controls (CMC) regulatory ...
Manufactured by:AOBiome Therapeutic, LLC based inCambridge, MASSACHUSETTS (USA)
We have completed six Phase 2 clinical programs and multiple pre-clinical programs. Over 1,000 patients have been enrolled in our clinical trials since 2016. All of those patients except for 28 in our open label pediatric study have been enrolled in randomized double-blind placebo controlled clinical trials evaluating both topical and intranasal routes of delivery of AOB with treatment durations ...
Manufactured by:Novo Nordisk A/S based inBagsværd, DENMARK
Our proprietary GalXC technology leverages a naturally occurring biologic process, ribonucleic acid interference, RNAi, to create therapies that silence disease-causing genes. This platform enables Dicerna to create molecules that can effectively interfere with the RNAi processes that lead to defective or misregulated proteins that cause disease. ...
Manufactured by:Vedanta Biosciences, Inc based inCambridge, MASSACHUSETTS (USA)
VE707 is a preclinical discovery program for the prevention of infection and colonization recurrence of several multi-drug resistant organisms (MDROs) including carbapenem-resistant Enterobacteriaceae (CRE), extended-spectrum beta lactamase producers (ESBL), and vancomycin-resistant Enterococci (VRE), which are some of the most common hospital-acquired ...
Manufactured by:eTheRNA based inNiel, BELGIUM
Therapeutic area: cancer vaccines; Systemic, intravenous (IV) ...
Manufactured by:Xstrahl based inNorcross, GEORGIA (US) (USA)
The SARRP FLASH system by Xstrahl exemplifies cutting-edge technology in preclinical radiation therapy. This system leverages the advanced FLASH-RT technique, which administers ultra-high dose rates of radiation, significantly surpassing conventional methods. This approach aims to effectively target tumors while minimizing damage to surrounding healthy tissues, making it pivotal for translational ...
Manufactured by:Enterome based inParis, FRANCE
New generation of oral bioactives to treat inflammatory and autoimmune diseases. EndoMimicsTM are first-in-class orally available bioactives drugs based on proteins secreted by the gut bacteria that act like human hormones or cytokines. Enterome expects partnering to play a central in generating value from its EndoMimics pipeline. ...
Manufactured by:BRIM Biotechnology, Inc. based inTaipei City, TAIWAN
BRM423 is targeted to treat severe corneal damage. BRM423 is novel synthetic peptide derived from human Pigment Epithelium-Derived Factor (PEDF). Its active ingredient is the same as the BRM421 for Dry Eye Syndrome, but it is targeted to treat severe corneal damage. Based on BRIM’s proprietary PDSP technology platform, BRIM intends to rapidly advance development of this novel treatment for ...