Show results for
Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
Pre Clinical Study Equipment Supplied In Europe
67 equipment items found
Manufactured by:InVivo BioTech Services GmbH a BRUKER Company based inHennigsdorf, GERMANY
Transient gene expression in mammalian cells provides reasonable amounts of recombinant proteins and antibodies which is sufficient for most applications. Nevertheless, when it comes to bulk productions for in vitro diagnostics or pre-clinical studies for biopharmaceutical development, the required amount of biological material often exceeds the ...
Manufactured by:Wolfmet based inManchester, UNITED KINGDOM
This efficiency, coupled with cost-effectiveness, has made Wolfmet 3D a game-changer in the design and manufacturing of collimators and shielding. Ongoing pre-clinical studies indicate that 3D printed tungsten collimators substantially enhance image quality. Wolfmet 3D's ability to produce standard as well as complex bespoke designs provides ...
Manufactured by:Pluristem Therapeutics Inc. based inHaifa, ISRAEL
PLX-Immune are cells that have been induced with tumor necrosis factor alpha (TNF-a) and interferon-gamma (IFN-g), to transiently alter their secretion profile. The product has been evaluated in pre-clinical studies and a peer-reviewed article in the journal Scientific Reports, from Nature, was published which examined the effect of the cells in ...
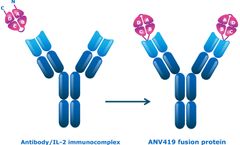
Manufactured by:Anaveon AG based inBasel, SWITZERLAND
The culmination of our research is ANV419, which is in clinical development. It is a rationally engineered fusion protein that is a stable, antibody-like molecule. ...
Manufactured by:CTIBiotech based inMeyzieu, FRANCE
Using the latest tools in bioengeneering, our researchers are now printing cancer tissues in 3D. These models can be used for highly significant pre-clinical trials and can be produced in 6/48/96 well plates for high throughput ...
Manufactured by:Medprin Biotech GmbH based inFrankfurt, GERMANY
100% absorbable within 7-14 days. Hemostasis in capillary, venous as well as small artery bleeding. StypCel™ works when ligation or other conventional methods are impracticable or ...
Manufactured by:BioCydex based inPoitiers, FRANCE
Vectisol® is dedicated to the preservation of organs, tissues and cells. This molecular complex limits the deleterious effects of the ischemia-reperfusion ...
by:XELTIS BV based inEindhoven, NETHERLANDS
The aXess graft is a restorative, synthetic, electrospun blood vessel for arteriovenous hemodialyisis access, overtime turning into a living vessel made of patient’s own ...
Manufactured by:ZIND Verfahrenstechnik based inMainz, GERMANY
At pilot stage, the primary membrane candidate has been chosen, as process parameters. Typically at this stage, the production method is being verified and production materials produced for toxicology or pre-clinical trials and predictable scalability is imperative. In order to facilitate the manufacturing life cycle of a successful therapeutic candidate, cGMP manufacturing needs to begin at ...
Manufactured by:MR Solutions based inGuildford, UNITED KINGDOM
The MRS*PET INSERT, designed by MR Solutions, facilitates simultaneous PET/MR imaging, enabling the acquisition of multi-parametric information from the same subject with precision. Built to integrate seamlessly with cryogen-free MR systems up to 9.4 Tesla, it offers flexibility in research settings due to its removable nature. This product can function independently of the MR system, effectively ...
Manufactured by:AstraZeneca based inCambridge, UNITED KINGDOM
IVX-121 targets respiratory syncytial virus (RSV), a major cause of viral pneumonia for which no vaccine has been FDA approved. IVX-121 incorporates a stabilized prefusion F antigen licensed from NIAID/NIH (DS-Cav1; Science 2019). RSV F is known to undergo major structural changes that allow viral entry into the host cell, and during that process, critical protective epitopes are lost. Protein ...
Manufactured by:Novo Nordisk A/S based inBagsværd, DENMARK
Our proprietary GalXC technology leverages a naturally occurring biologic process, ribonucleic acid interference, RNAi, to create therapies that silence disease-causing genes. This platform enables Dicerna to create molecules that can effectively interfere with the RNAi processes that lead to defective or misregulated proteins that cause disease. ...
Manufactured by:eTheRNA based inNiel, BELGIUM
Therapeutic area: cancer vaccines; Systemic, intravenous (IV) ...
by:XELTIS BV based inEindhoven, NETHERLANDS
Fully synthetic restorative pulmonary heart valve for Right Ventricular Outflow Tract reconstruction, overtime turning into a living heart valve made of patient’s own ...
Manufactured by:Enterome based inParis, FRANCE
New generation of oral bioactives to treat inflammatory and autoimmune diseases. EndoMimicsTM are first-in-class orally available bioactives drugs based on proteins secreted by the gut bacteria that act like human hormones or cytokines. Enterome expects partnering to play a central in generating value from its EndoMimics pipeline. ...
Manufactured by:2A Pharma ApS based inAalborg Øst, DENMARK
The global market for prophylactic HPV vaccines is likely to grow rapidly as more and more countries expand national vaccination programs to include HPV vaccinations not only for girls but also for boys. In addition, it is now estimated that 60-70% of oropharyngeal cancers are caused by HPV virus infections, opening up a discussion on HPV vaccinations for adults ...
Manufactured by:Brenus Pharma based inIssoire, FRANCE
An allogeneic first in class immunotherapy harnessing the patient immune system to fight immune tolerance and prevent cancer ...
Manufactured by:Kiadis Pharma NV based inAmsterdam, NETHERLANDS
K-NK00X: Kiadis is evaluating preclinical programs with K-NK-cell therapies for the treatment of hematologic and solid ...
Manufactured by:Kuros Biosciences A.G. based inSchlieren, SWITZERLAND
MagnetOs isn’t like other bone grafts. It grows bone even in soft tissue thanks to our unique NeedleGrip™ surface technology which provides traction for our body’s vitally important ‘pro-healing’ immune cells (M2 macrophages).*†6,7 This in turn, unlocks previously untapped potential to stimulate stem cells – and form new bone throughout the graft.*8-10 ...
Manufactured by:Sequana Medical NV based inSint-Denijs Westrem, BELGIUM
Direct Sodium Removal (DSR®) therapy is Sequana Medical’s novel and proprietary approach under development for the management of fluid overload due to heart failure. The sodium concentration in patients with fluid overload is in balance but there is too much sodium and too much fluid in the body. Sequana Medical’s approach is to remove excess sodium in patients with ...