Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Prostatic Equipment Supplied In Usa
335 equipment items found
Manufactured by:Exosome Diagnostics, Inc. based inWaltham, MASSACHUSETTS (USA)
The only exosome-based test that provides unique, actionable intelligence to help you decide if biopsy is necessary; independent of PSA and other standard of care (SOC) features. Connect with a representative in your area for more information today. ...
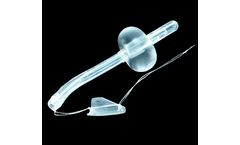
Manufactured by:SRS Medical based inN. Billerica, MASSACHUSETTS (USA)
The Spanner® Prostatic Stent is a temporary stent inserted into the urethra at the neck of the bladder to maintain urine flow. The Spanner benefits men with bladder outlet obstruction (BOO) by reducing elevated post void residual (PVR), improving voiding symptoms, and allowing for voluntary urination. ...
Manufactured by:UroLift System based inPleasanton, CALIFORNIA (USA)
UroLift Implant: the obstructing prostatic lobes are held apart by small permanent UroLift Implants that are deployed by the delivery device. Each implant is made with common implantable materials: nitinol, stainless steel, and PET suture. Typically, four implants are placed into the ...
Manufactured by:Veracyte, Inc. based inSouth San Francisco, CALIFORNIA (USA)
Providing clarity and confidence in treatment planning. The Decipher Prostate (Decipher Prostate Biopsy and Decipher Prostate RP) genomic classifier is a 22-gene, whole-transcriptome-developed test designed to help inform treatment decisions for men with localized prostate cancer at initial diagnosis and after surgical removal of ...
Manufactured by:ProUroCare Medical Inc. based inEden Prairie, MINNESOTA (USA)
ProUroScan is an advanced medical imaging system that uses an array of sensors mounted on a rectal probe, a central processing unit and software and image construction algorithms to provide a real time color image of abnormalities in the ...
Manufactured by:Pathnostics based inIrvine, CALIFORNIA (USA)
Prostate biopsies are performed when results from initial tests, like a prostate-specific antigen (PSA) blood test or digital rectal exam, indicate the possibility of prostate cancer. A range of tissue samples are extracted with a needle from the prostate gland and are analyzed under a microscope to identify cancerous cell ...
Manufactured by:Best NOMOS based inPittsburgh, PENNSYLVANIA (USA)
Fast, simple and precise prostate brachytherapy. Safe stabilization and precise seed ...
Manufactured by:Cleveland Diagnostics, Inc. based inCleveland, OHIO (USA)
IsoPSA Proceed to biopsy only when needed. IsoPSA is now included in NCCN® Prostate Cancer Early Detection Guidelines. IsoPSA can help reduce unnecessary prostate biopsies by up to 55%. Up to 75% of results from prostate biopsies performed in the US are negative for high-grade disease. By singling out PSA proteins that came from cancer cells, ...
Manufactured by:Prodeon Medical, Inc. based inSunnyvale, CALIFORNIA (USA)
Struggling with lower urinary tract symptoms related to benign prostatic hyperplasia (BPH)? Approximately, 60% of men over the age of 60 and up to 80% of men overall, will eventually develop BPH with LUTS, of which 30% will require ...
by:Aphios Corporation based inWoburn, MASSACHUSETTS (USA)
Prostate cancer is the most prevalent cancer among men, and it is the second leading cause of cancer death among men in the United States. More than 500,000 cases are diagnosed annually. In the U.S. this year, 1 in 6 males will develop the disease and 30,000 others will die of it. Although prostate cancer mostly affects elderly men, the number of younger men with ...
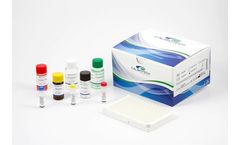
Manufactured by:Calbiotech Inc. based inEl Cajon, CALIFORNIA (USA)
Catalog number: PS235T. Product type: ELISA. Quantity: 96 Tests (12x8 breakable strip wells). Standard range: 2-50 ng/ml. Analytical Sensitivity: 2 ng/ml. Sample volume: 25 µl/well. Species: Human. Storage and Stability: Product should be stored at 2-8 °C. Precautions: For research use only. Not for use in diagnostic procedures. ...
Manufactured by:Exosome Diagnostics, Inc. based inWaltham, MASSACHUSETTS (USA)
The ExoDx Prostate is a simple, non-invasive urine test that can discriminate the risk of aggressive prostate cancer (defined as Gleason Score ≥7). This test is now available as an At-Home Collection Kit that can be initiated by an electronic order signed by the physician and sent directly to the patient’s home for ...
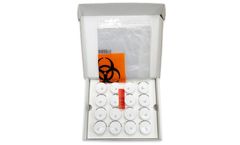
Manufactured by:Path-Tec, LLC based inMidland, GEORGIA (US) (USA)
Transport Box with Insert, (16) 10 percent NBF 20ml Vials with security seals, Biohazard Bag with Absorbent, Box ...
Manufactured by:Cancer Targeted Technology based inWoodinville, WASHINGTON (USA)
CTT1057 is an innovative 18F-labeled PET agent for prostate cancer that can be imaged within 2 hours of administration. CTT1057 will specifically and sensitively detect cancer that has escaped from the prostate as well as distant metastatic disease including bone metastases with greater precision and resolution than current standard of care. With uncertainty in ...
Manufactured by:Profound Medical Corp. based inMississauga, ONTARIO (CANADA)
Transurethral directional ultrasound for thermal ablation; water cooling of urethra and rectum: Sweeping ultrasound, continuous rotation. Capable of treating both large and small prostate volumes, anterior and posterior prostate tissue. Thermal protection of important anatomy. Closed-loop process control software: Real-time temperature feedback provides for ...
Manufactured by:miR Scientific, LLC based inNew York, NEW YORK (USA)
The science behind miR’s prostate and bladder cancer detection and classification technology that can help give healthcare providers, patients, and payors the greatest confidence in test results and care ...
Manufactured by:MDxHealth based inIrvine, CALIFORNIA (USA)
ConfirmMDx Addresses Prostate Biopsy Sampling Error and the False-Negative Biopsy Dilemma. “Rule-in” high-risk men who have had a previous negative biopsy result, may be harboring undetected cancer (a false-negative biopsy result), and therefore may benefit from a repeat biopsy and appropriate treatment. ...
Manufactured by:MDxHealth based inIrvine, CALIFORNIA (USA)
SelectMDx Helps Physicians Determine if a Patient is at Higher or Lower Risk for Prostate Cancer and Which Men Can Safely Avoid Biopsy. A non-invasive urine test (“liquid biopsy”), SelectMDx measures the expression of two mRNA cancer-related biomarkers (HOXC6 and DLX1).1 The test provides binary results that, when combined with the patient’s clinical risk ...
Manufactured by:Pathnostics based inIrvine, CALIFORNIA (USA)
The Guidance Pre Prostate Biopsy Rectal Swab (GPPBRS) test identifies antimicrobials that may be used prophylactically by using our patented Pooled-Antimicrobial Susceptibility Testing technology to determine the presence or absence of resistance genes. When an appropriate antimicrobial is used before and after the prostate biopsy procedure, the risk of infection ...
Manufactured by:Cancer Targeted Technology based inWoodinville, WASHINGTON (USA)
CTT’s lead therapeutic product, CTT1403, is a PSMA-targeted 177Lu-labeled agent for prostate cancer that can has superior binding characteristics, long circulation half-life, enhanced tumor uptake and unparalleled anti-tumor efficacy. CTT1403 will specifically deliver the radionuclide, 177Lu, minimizing toxic side effects and more effectively treating metastatic ...