- Home
- Equipment
- usa tennessee
- soft tissues
Show results for
Refine by
Soft Tissues Equipment Supplied In Usa Tennessee
13 equipment items found
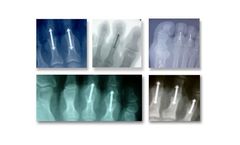
Manufactured by:Crossroads Extremity Systems based inMemphis, TENNESSEE (USA)
There are potential risks associated with the use of these devices, some of which include: allergic reaction to the implant material, fracture of the implant, soft-tissue complication (e.g., infection at the implant site, prolonged healing), and revision surgery. Refer to IFU for all contraindications, warnings, and risks. ...
Manufactured by:Active Implants LLC based inMemphis, TENNESSEE (USA)
The implant is made from polycarbonate-urethane (PCU) – a medical grade plastic. As a result of its unique materials and composite structure and design, it does not require fixation to bone or soft tissues. The NUsurface Implant mimics the function of the natural meniscus and redistributes loads transmitted across the knee ...
Manufactured by:Microport Orthopedics Inc. based inArlington, TENNESSEE (USA)
MicroPort Orthopedics acetabular cup systems have established clinical heritage built on over 25 years of clinical presence. Manufactured from titanium for biocompatibility and optimized stiffness, the market introduction of the Lineage® acetabular system delivered unsurpassed surgical flexibility for shell fixation and bearing material choice. The innovative internal shape is designed to ...
Manufactured by:Microport Orthopedics Inc. based inArlington, TENNESSEE (USA)
The Prime acetabular cup system is the next step in the evolution of the successful Dynasty® acetabular cup system. The system is optimized for a highly cross-linked ultra-high molecular weight polyethylene bearing surface, eliminating the compromises associated with modularity to accommodate alternative bearing surfaces. By focusing on a singular bearing surface, the shell has also been ...
Manufactured by:Arrowhead Medical Device Technologies, LLC based inCollierville, TENNESSEE (USA)
Commit to trying ARROW-LOK™ on at least five patients while closely following the recommended technique. As simple as the ARROW-LOK™ is and uncomplicated the operative technique may be, please expect to take at least five cases to become comfortable with the product, the operative technique and how the design interacts with the anatomy. Five (5) cases will give the surgeon the ...
by:In2Bones Global based inMemphis, TENNESSEE (USA)
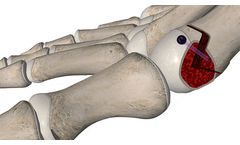
The AlloAid® DIP is a sterile, engineered Allograft. The Allograft is supplied in 2 sizes. The AlloAid® DIP complies with all FDA, AATB and state regulatory requirements for donor screening, recovery and ...
Manufactured by:Crossroads Extremity Systems based inMemphis, TENNESSEE (USA)
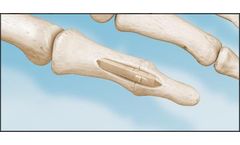
The MINIBunion is a minimally-invasive implant and procedure that spares the joint capsule and gives patients a walking recovery instead of being in a cast for several weeks. The minimally-invasive Minibunion® procedure allows bunion repair through a tiny 15mm incision on the side of the foot. The incision spares the joint capsule and is basically not visible after it heals. ...
by:In2Bones Global based inMemphis, TENNESSEE (USA)
“The indications for surgical management of radial head fractures are not well defined in the literature. Fragment size, number, degree of displacement and bone quality influence decision making regarding optimal management. Associated injuries and a block to motion are also important factors to consider. Good results have been reported after ORIF for selected noncomminuted displaced radial ...
Manufactured by:DT MedTech LLC based inMcMinnville, TENNESSEE (USA)
The Hintermann Series H3 Total Ankle Replacement System (Hintermann Series H3) is a non-constrained, mobile bearing three-component total ankle replacement system approved by the FDA. ...
Manufactured by:Medacta International based inCastel San Pietro, SWITZERLAND
Patient expectations are not as well fulfilled by TKA as by total hip replacement with fewer knee patients achieving a “forgotten joint” replacement. Studies show that around 20% of TKA patients are not satisfied[1, 2, 3]. Excessive A/P motion may result in anterior knee pain and continued swelling. In many P/S designs, the stabilizing mechanism only engages after 70-80° of ...
Manufactured by:Medacta International based inCastel San Pietro, SWITZERLAND
MOTO Medial offers uncompromised anatomical fit and compartment-specific coverage that accommodates the broadest range of patient anatomies. It features a patient specific gap balance and alignment technique with minimized and precise bone resections, without ligament releases. The implant design and instrumentation work together so that intraoperative decision making and flexibility are ...
Manufactured by:DT MedTech LLC based inMcMinnville, TENNESSEE (USA)
The Hintermann Series H2 Total Ankle Replacement System (Hintermann Series H2) is a semi-constrained, total ankle replacement system consisting of a talar component, tibial assembly component, and a PE inlay assembly component. Prof. Beat Hintermann developed the Hintermann Series H2 based on his more than 20 years of experience implanting his successful Hintermann Series H3® ankle outside ...
Manufactured by:Arrowhead Medical Device Technologies, LLC based inCollierville, TENNESSEE (USA)
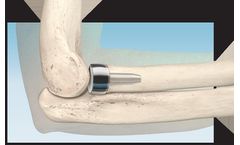
The ARROW-LOK implant features a three-dimensional arrow shape at each end joined by a solid shaft. The implants are available in multiple lengths and in 2 different angles. The implant is manufactured from stainless steel per ASTM ...