- Home
- Equipment
- north america
- therapeutic crawling device
Show results for
Refine by
Applications
- Ultrasound Therapy Machine
- Personal Ultrasound Machine for Pain
- Medical Device Solutions for Full Lifecycle
- Medical Device Solutions for Opportunity Realization
- Surface Acoustic Wave Treatment in Urology
- Intraoperative Radiation Therapy (IORT) System - Electron Therapy for Skin Cancer
- Intraoperative Radiation Therapy (IORT) System for Flash Radiotherapy
- Intraoperative Radiation Therapy (IORT) System for Pancreatic Cancer Treatment
- Intraoperative Radiation Therapy (IORT) System for Breast Cancer Treatment
- Intraoperative Radiation Therapy (IORT) System for Single-Fraction APBI
- Intraoperative Radiation Therapy (IORT) System for Surgical Oncologists
- Intraoperative Radiation Therapy (IORT) System for Radiation Oncologists
- Intraoperative Radiation Therapy (IORT) System for Hospital Administrators
- Medical Devices for Medical Professionals
- Medical Devices for Patients
- Medical Devices for Chronic Venous Insufficiency: Eliminating Pain
- Medical Devices for Breast Cancer-Related Lymphedema
- Medical Devices for Lipedema
- Medical Lasers Systems for Laser Treatments
- Medical Lasers Systems for Dental Lasers
- Medical Lasers Systems for Podiatry Lasers
- Medical Lasers Systems for Laser Acupuncture
- Medical Lasers Systems for Chiropractic Lasers
- Medical Lasers Systems for Physical Medicine
- Medical Lasers Systems for Laser Massage Therapy
- Medical Lasers Systems for Laser Physical Therapy
Therapeutic Crawling Device Equipment Supplied In North America
185 equipment items found
Manufactured by:MedSpark, LLC based inSan Luis Obispo, CALIFORNIA (USA)
Learning to crawl properly prior to walking is an important part of early childhood physiological development. However, several developmental disabilities can inhibit this process, including Cerebral Palsy, Spina Bifida, Downs Syndrome, Traumatic Brain Injury, and various physical abnormalities. Many therapeutic crawling devices ...
by:Winback Swims America based inWhite Plains, NEW YORK (USA)
The BACK 3SE model is the top of the WINBACK Tecar therapy range. It can be equipped with 5 types of Winback Tecar therapy (from 1.0 to 5.0). Very practical, its accessories cradle and mobile trolley allows easy movement between colleagues. It is also scalable for future innovations. ...
Manufactured by:NeuroMD based inNorth Miami Beach, FLORIDA (USA)
Micro USB Charge Port Center devices ordered between 10/03/2020 - 11/05/2021. Existing customer replacement part. Double-check your charge port to ensure you are ordering the correct ...
by:Winback Swims America based inWhite Plains, NEW YORK (USA)
The BACK 3SE model is the top of the WINBACK Tecar therapy range. It can be equipped with 5 types of Winback Tecar therapy (from 1.0 to 5.0). Very practical, its accessories cradle and mobile trolley allows easy movement between colleagues. It is also scalable for future ...
Manufactured by:.decimal based inSanford, FLORIDA (USA)
There are many advantages to the static delivery of a photon compensator. Because the entire tumor is receiving dose simultaneously, treatment times and monitor units are reduced significantly. This is especially beneficial for very large fields equating to a shorter time on the table and less radiation for your patient. Compensators are not for every patient, but when the right case presents ...
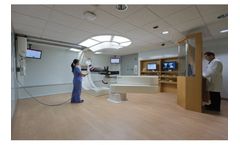
Manufactured by:Mevion Medical Systems based inLittleton, MASSACHUSETTS (USA)
The MEVION S250™ provides next-generation proton therapy. Built on the world’s only gantry-mounted proton accelerator, the MEVION S250 delivers a highly stable, uniform proton beam that is simple to deliver. The MEVION S250 has been treating patients since 2013 at multiple treatment sites across the ...
Manufactured by:Zepf Surgical Instruments based inBayport, NEW YORK (USA)
This simplified smart percussive therapy device prioritizes the essential features you need while maintaining the power and effectiveness of Theragun's deep muscle treatment. Ease discomfort, soothe tightness and tension, and recover faster in seconds. ...
Manufactured by:NasoNeb | Monaghan Medical Corporation based inPlattsburgh, NEW YORK (USA)
The NasoNeb® nebulizer cup and tubing should be replaced every 6-12 months; Does Not Include Compressor UnitFor use with the NasoNeb® Sinus Therapy System model ...
Manufactured by:Ex N’ Flex – Movement For Life! based inOttawa, ONTARIO (CANADA)
The Ex N’ Flex EF-100 has been designed for those who have little or no control over arm, shoulder and trunk movement. In passive mode, the arms and shoulder are rotated by the electrical motor in a slow predictable orbital motion. In active mode you can assist the motor to move the arm crank with your own muscles, thus building and maintaining strength and endurance in the arm and ...
Manufactured by:Novuson based inBothell, WASHINGTON (USA)
For those that prefer 5mm access, our 5mm Ultrastat LS will be capable of sealing and dividing vessels up to ...
Manufactured by:Saladax Biomedical, Inc. based inBethlehem, PENNSYLVANIA (USA)
BSA dosing results in sub-target 5-FU levels in the majority of patients. PK- guided dosing can move 5-FU levels into the target range. Optimal dosing of 5-FU is associated with increased efficacy and reduced ...
Manufactured by:X-Cel-X-Ray Corporation based inCrystal Lake, ILLINOIS (USA)
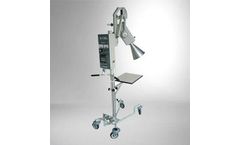
Easy-to-roll mobile unit. Removeable and adjustable table for treatment of hands and feet. Control box is mounted on the post featuring a pivot to enable viewing the controls from three different positions. Plastic tipped treatment cones, 1cm, 3cm, and 5cm at 12cm target to skin distance, are standard. Effective for treatment of eczema and psoriasis. ...
Manufactured by:Genadyne Biotechnologies, Inc. based inHicksville, NEW YORK (USA)
UNO Single Use Negative Pressure Wound Therapy System. Lightweight – 400 grams or 0.88 pounds, Simple, easy and intuitive interface, Two pressure settings: Continuous at 80mmHg or 125mmHg, Variable at 80mmHh / 30mmHg or 125mmHg / 30mmHg, 3 Alerts: Leakage, Canister Full, Critical ...
Manufactured by:Saluda Medical Pty Ltd. based inBloomington, MINNESOTA (USA)
Evoke is the first and only spinal cord stimulation (SCS) technology designed to measure your body’s unique response to therapy and provide the precise amount of stimulation necessary to combat your pain. Evoke automatically makes millions of small adjustments throughout the day to deliver consistent therapy so you can focus less on your pain and focus more on living your life. ...
Manufactured by:AMREX, Trademark of Amrex-Zetron Inc. based inParamount, CALIFORNIA (USA)
The MS324C is designed for the application of low volt ac muscle stimulation. The MS324C is dual channel, four pad, low voltage electrical muscle stimulator that produce pulsation, tetanize, surge, and reciprocal output. The MS324C features the patient treatment stop switch. Designed to promote patient assurance, the treatment stop switch allows the patient to discontinue stimulator output at the ...
by:Theralase Technologies Inc. based inToronto, ONTARIO (CANADA)
Application: Used in the treatment of the majority of nerve muscle and joint conditions, as it covers a significant tissue surface area. Choice of four different power levels to best suit your practice and your pocket book. Treatment Ares: 20 ...
Manufactured by:ProMed Specialties based inHuntington Valley, PENNSYLVANIA (USA)
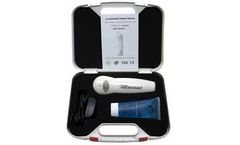
Three Treatment Modes (Low, Medium and High). Easy-to-use Push Button Control. Packaged in attractive PVC case. Sold complete with AC Adapter, Conductive Gel, Manual, and carrying ...
Manufactured by:Novartis International AG based inBasel, SWITZERLAND
Our investigational gene therapy, GT005, is designed to restore balance to a part of our immune system called the complement ...
Manufactured by:Mevion Medical Systems based inLittleton, MASSACHUSETTS (USA)
The MEVION S250mx™ is a scalable proton therapy solution with two, three or four room options. Because of Mevion’s unique core technology, each treatment room is fully independent. This gives you the flexibility to add rooms as patient volume grows, allowing you to adapt to increasing demand, minimize financial risk, and maximize your clinical and operational ...
Manufactured by:Orgenesis Inc. based inGermantown, MARYLAND (USA)
We leverage closed, automated processes that are validated for reliability and ...