Refine by
Transplantable Organs Equipment & Supplies
38 equipment items found
Manufactured by:Precise Bio based inWinston-Salem, NORTH CAROLINA (USA)
Precise Bio’s innovative 4D-bio-fabrication technology is a true platform for innovation. It comprises cell expansion, biomaterials, processes, printing technology and other required critical technologies. Key advantages over other biofabrication approaches are the ability to generate complex tissues in a highly reproducible manner and to apply lessons learned from the fabrication ...
Manufactured by:CatchGene Co., Ltd based inNew Taipei City, TAIWAN
But in the past two decades, scientists realized that not only normal cells but also cancer cells, embryo or even transplant organs will release their DNA into blood stream. Therefore, how to capture those very rare and small cfDNA becomes very critical for Precision Medicine. ...
Manufactured by:Bridge to Life™ Ltd. based inNorthbrook, ILLINOIS (USA)
The VitaSmart Hypothermic Oxygenated Perfusion System, developed by Bridge to Life™ Ltd., is designed to enhance the organ transplantation process by facilitating hypothermic oxygenated perfusion. This system is intended for application in countries that accept the CE mark, despite currently lacking FDA approval in the US. Bridge to Life™ also offers ...
Manufactured by:Bridge to Life™ Ltd. based inNorthbrook, ILLINOIS (USA)
The VitaSmart™ Hypothermic Oxygenated Machine Perfusion System is designed to revolutionize organ transplantation by facilitating the application of hypothermic oxygenated perfusion (HOPE) protocols. This system simplifies the perfusion process with its user-friendly setup, requiring minimal human resources for monitoring. While the system's use is not yet ...
Manufactured by:United Therapeutics Corporation based inSilver Spring, MARYLAND (USA)
Perfusion recellularization begins with organ scaffolds created by perfusion decellularization. These scaffolds are capable of receiving and incorporating a variety of cell types, depending on the organ scaffold utilized. Perfusion decellularization enables organ scaffolds to retain their native vasculature and so cells are introduced through existing vascular ...
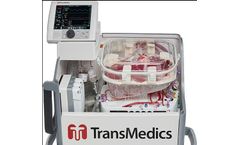
Manufactured by:TransMedics, Inc. based inAndover, MASSACHUSETTS (USA)
Successful Completion of 300 Patient US FDA Pivotal Trial for Expanded Criteria and DCD Donor Livers. A prospective, pivotal, randomized trial to evaluate the effectiveness of the OCS Liver to preserve and assess donor livers intended for ...
Manufactured by:Infomed SA based inMeinier, SWITZERLAND
TPE allows to remove patient’s plasma from the blood and to replace it with fluids which do not contain the large substances, such as antibodies, targeted to be removed. It applies to a wide range of acute diseases but more rarely to chronic ones due to the adverse effects of the replacement fluids, human albumin or fresh frozen plasma, ...
Manufactured by:Universal Cells Inc. based inSeattle, WASHINGTON (USA)
Combining class I and class II engineering to create Universal Donor Cells (UDCs) that are appropriate for developing numerous differentiated cell ...
Distributed by:Air Equipment Company based inLouisville,, KENTUCKY (USA)
Air Equipment Company is the exclusive representative for Precision Air Products, manufacturers of laminar flow diffusers and integrated ...
Manufactured by:Infomed SA based inMeinier, SWITZERLAND
Like TPE, cascade filtration and DFPP allow to remove the larger substances from the blood, such as antibodies, fibrinogen or LDL-cholesterol. Its interest remains in the fact that the plasma is filtered and then returned to the patient, avoiding or minimizing the need for replacement fluids. Thus, cascade filtration/DFPP applies to both acute and chronic ...
Manufactured by:Jiangsu OLYMSPAN Equipment Technology Co., LTD based inChangzhou, CHINA
Using the excellent mechanical properties of carbon fiber composite materials, it is used in the manufacture of orthopedics and organ transplantation and other medical devices, as well as the manufacture of prosthetics, orthotics and other rehabilitation products. ...
Manufactured by:Biotest AG based inDreieich, GERMANY
IgG Next Generation is a new development of a polyvalent immungiobulin (NIG) for the treatment of immunedeficiencies and auto immune diseases. IgG Next Generation is manufactured with a new and innovative production process and will be the main product of the new manufacturing site Biotest Next ...
Manufactured by:Biotest AG based inDreieich, GERMANY
IgG Next Generation is a new development of a polyvalent immunglobulin (IVIG) for the treatment of immunedeficiencies and auto immune diseases. IgG Next Generation is being manufactured with a new and innovative production process and will be the main product of the new manufacturing site Biotest Next ...
Manufactured by:Beldico based inMarche-en-Famenne, BELGIUM
Transporting organs and tissues for transplantation in complete safety with Beldico sterile bags for organ ...
Manufactured by:Tonix Pharmaceuticals Holding Corp. based inChatham, NEW JERSEY (USA)
TNX-1500 incorporates the antigen binding fragment (Fab) region of hu5c8 (recombinant monoclonal antibody to CD40-ligand), that has been extensively characterized at the molecular level in complex with the CD40-ligand. TNX-1500 is being studied and evaluated for its role in preventing or treating organ transplant rejection and for treating autoimmune ...
Manufactured by:CuraMedical B.V. based inAssendelft, NETHERLANDS
Oxidised Renegerated Cellulose Natural, plant based cellulose is dissolved and extruded as a continuous fibre (regeneration). The fabric made from the fibre is very uniform in chemical composition and its oxidation results in less variation in stability and absorbility of the material compared to cotton based products. CuraCel® Absorbable Haemostat is a sterile product made of oxidized ...
by:Kephera Diagnostics, LLC based inFramingham, MASSACHUSETTS (USA)
With support from a National Institutes of Health grant, Kephera is developing a new point-of-care test for Chagas disease. Kephera scientists have identified and are purifying parasite components which promise to yield a highly sensitive and specific test. Together with our academic collaborators in the U.S. and Latin America, we are evaluating the performance of the test as it is developed in ...
Manufactured by:Celdara Medical, LLC based inLebanon, NEW HAMPSHIRE (USA)
Celdara Medical gives hope and health to patients by transforming academic innovations into medicines that cure the world’s most challenging ...
Manufactured by:Optibio Co., Ltd. based inCheongju-si, SOUTH KOREA
Other stressors may include viral infections, trauma, surgery, pancreatitis, burns, shocks associated with cardiac arrest, and acute organ transplant rejection. PCT tests are conducted to detect the presence of a bacterial infection in its early stages and are also, in crucially ill patients, used to guide antibiotic treatment by differentiating between bacterial ...
Manufactured by:United Therapeutics Corporation based inSilver Spring, MARYLAND (USA)
Miromatrix is developing bioengineered organs for transplantation, and our technology can be applied across the spectrum of donor needs. We are currently focused on bioengineering transplantable kidneys (MiroKidney) and livers (MiroLiver), but we believe our technology can be used to engineer other in-demand organs, such as ...