Refine by
Trauma Tumor Equipment & Supplies
18 equipment items found
Manufactured by:Matexcel based inShirley, NEW YORK (USA)
It can be used in orthopedic clinical research, and gradually to the plastic surgery and cosmetic surgery, dental, repair of bone defects and bone fusion caused by trauma, tumor, inflammation, bone disease, etc. And other areas of development.. Morphology & Appearance : White strip or column. Particle Size : Columnar: ?6, 1-10mm height;?8, 1-10mm height;or ...
Manufactured by:Neo Medical Sa based inVillette, SWITZERLAND
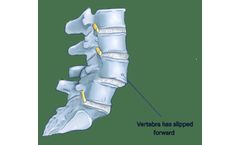
This condition occurs as a consequence of the general aging process in which the bones, joints, and ligaments in the spine become weak and less able to hold the spinal column in alignment, but may also be caused by stress fractures, or congenital abnormalities, and in rare cases from a tumor or trauma. ...
Manufactured by:Neuro France Implants based inLa Ville aux Clercs, FRANCE
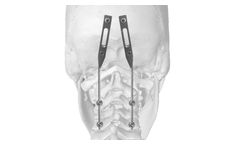
J.O.C. system allows to perform a posterior occipito-cervical ...
Manufactured by:NuVasive, Inc. based inSan Diego, CALIFORNIA (USA)
The XLIF Corpectomy technique allows surgeons to treat corpectomy patients through a reproducible, minimally disruptive exposure that provides direct visualization to the affected ...
Manufactured by:Spine Wave, Inc. based inShelton, CONNECTICUT (USA)
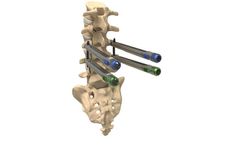
Next gen minimally invasive pedicle screw system designed to treat degeneration, deformity, tumor, or trauma of the thoracolumbar ...
Manufactured by:Biedermann Motech GmbH & Co. KG based inVillingen, GERMANY
Enabling advanced techniques in percutaneous ...
Manufactured by:ChoiceSpine LLC based inKnoxville, TENNESSEE (USA)
The ChoiceSpine THUNDERBOLT™ Minimally Invasive Pedicle Screw System is intended to provide immobilization and stabilization of spinal segments in skeletally mature patients as an adjunct to fusion for degenerative disc disease, spondylolisthesis, trauma, spinal stenosis, deformities, tumor, and/or ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Conventional allografts are used for various procedures to fill bony voids or gaps in a patient's skeletal system, restore segmental bone loss due to trauma, osteoporosis, removal of tumors or to aid in the surgical correction for a deformity. These grafts provide a scaffold for bone ingrowth to allow for remodeling with the patient’s own bone. These grafts ...
Manufactured by:icotec ag based inAltstätten, SWITZERLAND
Recognizing what usually remains hidden; The rough Ti-iT® titanium coating and the radiolucent BlackArmor® material make the KONG®-C VBR M vertebral body replacement a unique implant for the cervical spine. In particular, the KONG®-C VBR M paves the way for new perspectives and treatment approaches in tumor/radiation ...
Manufactured by:Neuro France Implants based inLa Ville aux Clercs, FRANCE
The PM BUTTERFLY® system is intended to provide osteosynthesis between two or more cervical vertebrae thanks to a plate and cervical ...
Manufactured by:Escalon Ophthalmics based inLake Success, NEW YORK (USA)
Unparalleled in every regard: image quality, advanced tools including automated quantitative angle analysis, sulcus-to-sulcus probe alignment, and more. Elegant, intuitive, ...
by:Aurora Spine, Inc. based inToronto, ONTARIO (CANADA)
The ZIP ULTRA implant is designed for stabilization and load sharing in T1-S1 thoracolumbar fusion procedures, specifically for the treatment of degenerative disease, lumbar spinal stenosis, spondylolisthesis, trauma, and/or tumor. The proprietary ZIP ONE-STEP™ locking mechanism eliminates the use of a set screw. Each ZIP ULTRA implant features a large ...
by:Aurora Spine, Inc. based inToronto, ONTARIO (CANADA)
The ZIP LP implant is designed for stabilization and load sharing during T1-S1 thoracolumbar fusion procedures, specifically for the treatment of degenerative disc disease, lumbar spinal stenosis, spondylolisthesis, trauma, and/or tumor. The proprietary ZIP ONE-STEP™ locking mechanism eliminates the use of a set screw. Each ZIP LP implant features a large ...
by:Aurora Spine, Inc. based inToronto, ONTARIO (CANADA)
The ZIP 51 implant is designed for stabilization and load sharing during T1 - S1 thoracolumbar fusion procedures, specifically for the treatment of degenerative disc disease, lumbar spinal stenosis, spondylolisthesis, trauma, and/or tumor. The proprietary ZIP ONE-STEP™ locking mechanism eliminates the use of a set screw. Each ZIP 51 implant features a large ...
Manufactured by:Alliance Spine based inSan Antonio, TEXAS (USA)
Anterior cervical plate system with components manufactured from Ti-6Al-4V titanium ...
Manufactured by:Xtant Medical based inBelgrade, MONTANA (USA)
The Axle Interspinous Fusion System is designed to provide spinal stability for lumbar fusion procedures, including the treatment of degenerative disc disease, spinal tumors and trauma. ...
Manufactured by:Meticuly Co., Ltd. based inPathumwan District, THAILAND
Our patient-specific Mid-face Reconstruction Mesh is a patient-specific titanium mesh implant serving to replace defective bone lost in the cranio-maxillofacial region from trauma, congenital condition, or tumors. The implant’s contour is designed anatomically, based on CT scan data, to cover the bony void with overlapping margin. The implant has inbuilt ...
Manufactured by:Genesis Medical Plastics based inCypress, TEXAS (USA)
The Centers for Disease Control (CDC) define trauma as any wound or injury resulting from the application of force or violence to a living body. Under that definition, medical professionals face an exhaustive range of complications. Among the most common are bone fractures or conditions that attack bone tissue, such as tumors. Trauma fixation ...