- Home
- Equipment
- asia middle east
- clinical samples
Show results for
Refine by
Locations
- Asia & Middle East
- Afghanistan
- Armenia
- Azerbaijan
- Bahrain
- Bangladesh
- Bhutan
- Brunei Darussalam
- Cambodia
- China
- East Timor
- Georgia
- Guam
- India
- Indonesia
- Iran
- Iraq
- Israel
- Japan
- Jordan
- Kazakhstan
- Kuwait
- Kyrgyzstan
- Laos
- Lebanon
- Malaysia
- Maldives
- Mongolia
- Myanmar
- Nepal
- North Korea
- Oman
- Pakistan
- Palestinian Territories
- Philippines
- Qatar
- Russia
- Saudi Arabia
- Singapore
- South Korea
- Sri Lanka
- Syria
- Taiwan
- Tajikistan
- Thailand
- Turkmenistan
- United Arab Emirates
- Uzbekistan
- Vietnam
- Yemen
Clinical Samples Equipment Supplied In Asia Middle East
13 equipment items found
Manufactured by:Cnpair Biotech Co., Ltd. based inHangzhou City, CHINA
Recommended Usage: Coating. Recommended Pairing: 2019-nCoV NP antibody (7E9). Features: High effectivity in virus test of clinical ...
Manufactured by:KogeneBiotech based inGeumcheon-gu, SOUTH KOREA
PowerChek Respiratory Virus Real-time PCR Kit series provide the fast and accurate testing solution, and the kits are intended for the qualitative detection and discrimination of major respiratory viruses in clinical samples. KogeneBiotech is engaged with relevant organizations in Korea, to supply the test kits and offer additional respiratory viral detection ...
Manufactured by:KogeneBiotech based inGeumcheon-gu, SOUTH KOREA
The differentiation of Mycobacterium tuberculosis (TB) from non tuberculous mycobacteria (NTM) is of primary importance for infection control and choice of antimicrobial therapy.PowerChek TB/NTM Real-time PCR Kit seriesis designed to simultaneously detect the specific genes for TB and NTM using multiplex Real-time PCR assay. This kit allows for the qualitative detection and discrimination of TB ...
Manufactured by:NEST Biotechnology Co., Ltd. (NEST) based inNew District, CHINA
Its core function is to securely, reliably, and efficiently integrate a drug prefilled cartridge with NEST TSA disposable pen injector, forming the final drug-device combination product. This solution supports critical performance validation and clinical sample preparation prior to drug ...
by:Lytech Co. Ltd based inMoscow, RUSSIA
Reagent Kit POLYVIR SARS-CoV-2 is intended for qualitative detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA by real-time reverse transcription-polymerase chain reaction (real-time RT-PCR) in nucleic acid samples, isolated from clinical samples (nose, nasopharyngeal and oropharyngeal swabs, bronchoalveolar lavage, ...
Manufactured by:Jiangsu Bioperfectus Technologies Co., Ltd. based inShanghai, CHINA
(referred to as "Bioperfectus" below) is an in vitro diagnostic medical device, which integrates cutting-edge mechanical, electronic and software technologies to enable automated extraction of nucleic acids from samples with a modular structure - the equipment can extract nucleic acids from 1-96 samples in three independently operating modules at the same time. ...
Manufactured by:Celemics, Inc. based inSeoul, SOUTH KOREA
Celemics’ BRCA 1,2 Target Enrichment Panel is designed to enrich whole coding sequences (CDS) of the BRCA1 and BRCA2 genes including UTR, promoter, and +/- 40 bases of CDS to detect the variants in the splicing ...
Manufactured by:RNAssist based inCambridge, UNITED ARAB EMIRATES
Major deficiencies of current Virus Transport Media (VTM’s) are that they are variously toxic, incompletely inactivate viruses or are poor stabilisers of RNA and proteins, precluding their use for home-testing, transport, storage and safe handling. We have completed development of a solution that uniquely allows the inactivation and preservation of viruses at the point of sampling, whatever ...
Manufactured by:Panagene Inc. based inDaejeon, SOUTH KOREA
According to the WHO report (2008), human papillomavirus (HPV) causes virtually 100% of cases of cervical cancers. There are more than 100 genotypes of HPV reported currently, and HPV consists of double helix circular DNA with ca. 7,900 bps. Compared to the traditional Pap smear test, HPV genotyping has several advantages; 1) high accuracy of determining existence of HPV viruses, 2) genotyping of ...
Manufactured by:Pacific Edge Ltd based inDunedin,, NEW ZEALAND
Cxcolorectal is a prognostic gene signature for patients diagnosed with stage II or stage III colorectal cancer. The test predicts the aggressiveness of the tumour, allowing physicians to make the best decision regarding treatment for the ...
Manufactured by:Bioneer Corporation based inDaedeok-gu, SOUTH KOREA
ExiStation™ Universal Molecular Diagnostic System is an innovative semi-automated real-time qPCR based molecular diagnostic system. ExiStation™ is a workflow platform consisting of Max. 3 ExiPrep™ 16 Dx and one Exicycler™ 96. This unique composition allows simultaneous diagnosis of various diseases regardless of target and sample type. ExiStation™ is a pipetting-free ...
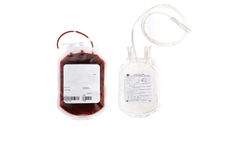
Manufactured by:Zhejiang PerfectSeal New Material Technology Co., Ltd. based inNingbo City, CHINA
A blood bag is a sterile, disposable container used for the collection, storage, and transfusion of blood and blood products. A 450ml blood bag is a single unit that can hold up to 450 milliliters of blood. The bag is made of a flexible, biocompatible plastic material that allows for safe and efficient collection, storage, and transportation of blood. The bag is designed with multiple ports and ...
Manufactured by:Infitek Co., Ltd. based inJinan, CHINA
Blood gas electrolyte analyzer includes blood gas measuring items (PH, PCO2, PO2). It is used to detect partial pressure of Carbon Dioxide (PCO2),and partial pressure of Oxygen (PO2) ...