- Home
- Equipment
- asia middle east
- diagnostic mobile ecg kit
Refine by
Locations
- Asia & Middle East
- Afghanistan
- Armenia
- Azerbaijan
- Bahrain
- Bangladesh
- Bhutan
- Brunei Darussalam
- Cambodia
- China
- East Timor
- Georgia
- Guam
- Hong Kong
- India
- Indonesia
- Iran
- Iraq
- Israel
- Japan
- Jordan
- Kazakhstan
- Kuwait
- Kyrgyzstan
- Laos
- Lebanon
- Malaysia
- Maldives
- Mongolia
- Myanmar
- Nepal
- North Korea
- Oman
- Pakistan
- Palestinian Territories
- Philippines
- Qatar
- Russia
- Saudi Arabia
- Singapore
- South Korea
- Sri Lanka
- Syria
- Taiwan
- Tajikistan
- Thailand
- Turkmenistan
- United Arab Emirates
- Uzbekistan
- Vietnam
- Yemen
Diagnostic Mobile Ecg Kit Equipment Supplied In Asia Middle East
47 equipment items found
Manufactured by:Wuxi Donglin Sci & Tech Development Co.,Ltd. based inBC V1W 4V3 Canada, CHINA
This Product name:Alpha-fetoprotein(AFP)quantitative diagnostic kit, Catalog number:TDL-AFP-Hu, Size:96T, Price:$240, Quality guarantee period:for 12 ...
Manufactured by:Sorbchem India Pvt Ltd based inVadodara, INDIA
Sorbchem India's Desiccant Tablets are designed to remove moisture from the top area of container or bottle during the time of tablets packaging. These tablets are ideal for blister packaging, MVTR testing, and use with diagnostics kits, electronic, and consumer packaging in pharmaceutical, nutraceuticals, and electronics ...
by:Pars Isotope Co based inTehran, IRAN
The PARSTEC II generator, manufactured by Parsisotope, is a crucial product in the realm of nuclear medicine. Designed for the extraction of technetium-99m (Tc-99m) in the form of a sodium pertechnetate solution, it serves as an essential tool for labeling SPECT diagnostic radiopharmaceutical kits. It facilitates the diagnosis of various cancers and diseases. Tc-99m is favored in medical ...
Manufactured by:Dotz Nano Ltd. based inKefar-Sava, ISRAEL
Dotz diagnostics test kit helps contagion prevention and supports the resuming of economic and social activities. Simultaneous 96 test (per device), the results are visible after approximately 30 ...
Manufactured by:Changzhou Munk Foam Technology Co.,Ltd based inChangzhou City, CHINA
Our Nylon Flocked Swabs feature perpendicular nylon fibers that optimize specimen collection and elution into transport media. The swabs also feature a molded breakpoint that allows you to safely and easily break off the swabstick, and several breakpoint options are available for different ...
Manufactured by:Hybribio based inKowloon Bay, HONG KONG
The High-risk HPV GenoArray Diagnostic Kit is crafted specifically for the qualitative detection of 14 prominent high-risk human papillomavirus (HPV) genotypes, including types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68. This kit utilizes a universal probe capable of indicating other HPV infections, making it a versatile tool for comprehensive HPV screening. It features an ...
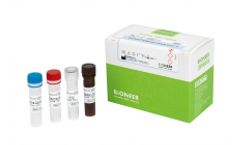
Manufactured by:Bioneer Corporation based inDaedeok-gu, SOUTH KOREA
AccuPower SARS-CoV-2 Variant ID2 Real-Time RT-PCR Kit(Mastermix type) is an in vitro diagnostic kit that helps diagnose SARS-CoV-2 variants. The spike gene (S gene) is the key region to monitor the mutants with the recent arising from emergence variants from SARS-CoV-2. The kit is able to detect 5 genes about S gene mutation hot-spots in 2 tubes. Sputum, nasopharyngeal swab, and oropharyngeal ...
Manufactured by:Sansure Biotech Inc. based inChangsha, CHINA
Mycobacterium tuberculosis virus (TB) is a pathogenic bacterium that causes tuberculosis. It is likely to infect all human tissues and organs, especially the lungs to cause pulmonary tuberculosis. Early diagnosis and treatment are important for effective control of tuberculosis. In recent years, with the development of molecular biology, nucleic acid fluorescence quantitative PCR method based on ...
Manufactured by:Wuhan EasyDiagnosis Biomedicine Co., Ltd based inWuhan, CHINA
FAST : 34 mins to get PCR result. PERFORMANCE : LOD: 200 copies/mL , CV≤5%. RELIABLE : Reagent freeze-drying ,can be transported at room ...
Manufactured by:KH Medical based inPyeongtaek-si, SOUTH KOREA
RADI Brucella spp Detection Kit is used for qualitative detection of Brucella in human whole blood cultured sample by using RADI Real Time PCR ...
Manufactured by:Bioneer Corporation based inDaedeok-gu, SOUTH KOREA
AccuPower SARS-CoV-2 Variant ID2 Real-Time RT-PCR Kit(Mastermix type) is an in vitro diagnostic kit that helps diagnose SARS-CoV-2 variants. The spike gene (S gene) is the key region to monitor the mutants with the recent arising from emergence variants from SARS-CoV-2. The kit is able to detect 5 genes about S gene mutation hot-spots in 2 tubes. Sputum, nasopharyngeal swab, and oropharyngeal ...
Manufactured by:Sansure Biotech Inc. based inChangsha, CHINA
15 High-risk HPV DNA Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the detection of high-risk HPV (type 16, 18, 31, 33,35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68) present in exfoliated cells from females’ cervix. The test results can be used as an aid in the diagnosis of a high-risk HPV infection. ...
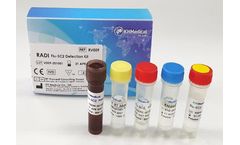
Manufactured by:KH Medical based inPyeongtaek-si, SOUTH KOREA
Fast Result and Easy to Use ! Get the result within 100 min for all suspected cases for Influenza A and/or Influenza B and/or SARS-CoV-2 (COVID-19)! The RADI Flu-SC2 Detection Kit is based on Real-time RT-PCR technology utilizing reverse-transcriptase(RT) reaction to convert RNA into complementary DNA (cDNA). It is intended for the presumptive qualitative detection of nucleic acid from the ...
Manufactured by:OPTOLANE Technologies Inc. based inSeongnam-si, SOUTH KOREA
The Genoplexor™ COVID-19 Detection Kit is an in vitro diagnostic kit designed for real-time reverse transcription polymerase chain reaction test intended for the qualitative detection of SARS-CoV-2 (RdRP gene, S gene) RNA in human samples such as human upper respiratory (oropharyngeal swab and nasopharyngeal swab) from individuals with suspected or confirmed respiratory ...
Manufactured by:Sansure Biotech Inc. based inChangsha, CHINA
This High-risk HPV DNA (Genotype) Diagnostic Kit (PCR-Fluorescence Probing) is an in vitro nucleic acid amplification test for the detection of high-risk HPV (type 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68) present in exfoliated cells from females’ cervix. The test results can be used for identification of HPV ...
Manufactured by:Trivitron Healthcare based inChennai, INDIA
Labsystems Diagnostics' Neonatal Biotinidase kit is for enzymatic determination of biotinidase enzyme activity from blood samples dried on filter paper, with fluorometric detection, intended for newborn screening for biotinidase ...
Manufactured by:Shenzhen AIVD Biotechnology Co. , LTD. based inCheektowaga, NEW YORK (USA)
The CRP Test is a fluorescent lateral flow immunoassay. The test consists of: 1) a conjugate pad containing CRP antibody conjugated with fluorescent microspheres and a control antibody (goat anti-rabbit IgG antibody) conjugated with fluorescence microspheres, 2) a nitrocellulose membrane strip containing a test line (T line) and a control line (C line). The T line is pre-coated with another CRP ...
Manufactured by:Shenzhen AIVD Biotechnology Co. , LTD. based inCheektowaga, NEW YORK (USA)
The FOB Rapid Test is a one-step chromatographic sandwich immunoassay. The test consists of: 1) a conjugate pad containing HB monoclonal antibody conjugated with colloidal gold. 2) a nitrocellulose membrane strip containing a test line (T line) and a control line (C line). The T line is pre-coated with another HB antibody, and the C line is pre-coated with a control line antibody (goat-anti-mouse ...
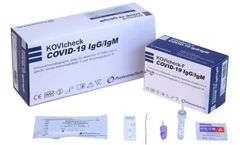
Manufactured by:PROTIA. iNC, formely known as ProteomeTech Inc. based inSeoul, SOUTH KOREA
KOVIcheck COVID-19 IgG/IgM is an in vitro diagnostic kit for use in the qualitative analysis of SARS-CoV-2 specific Immunoglobulin G (SARS-CoV-2 IgG) and Immunoglobulin M (SARS-CoV-2 IgM) in human serum, plasma and venous whole blood samples using Immunochromatography method. It aids diagnosis of COVID-19 infection in patients with clinical symptoms and provides only a screening test ...
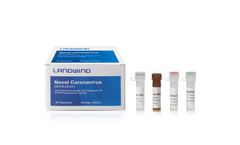
Manufactured by:Shenzhen Landwind Medical Co., Ltd based inShenzhen, CHINA
The genetic material of COVID-19 is RNA. The principle of the RT-PCR method is the specific match between the primers and probes and the specific region of COVID-19 RNA. Once the RNA of the virus is detected, the result will be "positive". In terms of Methodology, the nucleic acid detection technology is the most direct and the most intrinsical pathogenicity evidence. It is better than ...