- Home
- Equipment
- asia middle east
- inflammatory diseases
Refine by
Locations
- Asia & Middle East
- Afghanistan
- Armenia
- Azerbaijan
- Bahrain
- Bangladesh
- Bhutan
- Brunei Darussalam
- Cambodia
- China
- East Timor
- Georgia
- Guam
- India
- Indonesia
- Iran
- Iraq
- Israel
- Japan
- Jordan
- Kazakhstan
- Kuwait
- Kyrgyzstan
- Laos
- Lebanon
- Malaysia
- Maldives
- Mongolia
- Myanmar
- Nepal
- North Korea
- Oman
- Pakistan
- Palestinian Territories
- Philippines
- Qatar
- Russia
- Saudi Arabia
- Singapore
- South Korea
- Sri Lanka
- Syria
- Taiwan
- Tajikistan
- Thailand
- Turkmenistan
- United Arab Emirates
- Uzbekistan
- Vietnam
- Yemen
Inflammatory Diseases Equipment Supplied In Asia Middle East
15 equipment items found
Manufactured by:Bilix Co., Ltd. based inYongin, SOUTH KOREA
Many inflammatory diseases are associated with reactive oxygen species(ROS) in the body. The bilirubin nanoparticle has strong antioxidant, ROS scavenging, and immune modulating properties which effectively eliminate the disease-causing ...
Manufactured by:DxGen Corp. based inGunpo-si, SOUTH KOREA
Test Kit is for In-vitro diagnostic (IVD) use to determine C-reactive protein (CRP) quantitatively from human blood and serum/plasma. It can be user to diagnosis and follow-up for patients with infectious and non-infectious inflammatory diseases. ...
Manufactured by:DxGen Corp. based inGunpo-si, SOUTH KOREA
Test Kit is for in-vitro diagnostic (IVD) use to determine C-reactive protein (CRP) quantitatively in serum/plasma, or whole blood from a human. It provides information for the diagnosis, therapy, and monitoring of inflammatory ...
Manufactured by:Mesoblast Ltd. based inMelbourne, AUSTRALIA
Remestemcel-L is being developed for inflammatory diseases in children and adults including the treatment of acute graft versus host disease (aGVHD), a potentially life-threatening complication of an allogeneic bone marrow transplant (BMT). Currently, there are no products currently approved in the United States for treatment of ...
Manufactured by:Krishgen Biosystems based inMumbai, INDIA
Abnormally high levels of TNF have also been linked to a wasting syndrome that causes the body to drastically lose weight at an unhealthy rate. This is often common in cancer patients.Therefore, many inflammatory diseases have been treated by using a TNF inhibitor. ...
Manufactured by:SHL Medical AG based inZug, SWITZERLAND
The establishment of the Molly platform signifies SHL’s commitment to further addressing the varying needs of our customers and their patients. Introduced in 2010, Molly is SHL’s first preconfigured autoinjector designed to help pharmaceutical companies reduce initial investments and expedite development ...
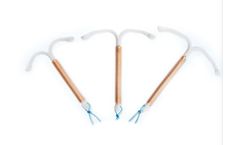
Manufactured by:SMB Corporation Of India based inMumbai, INDIA
Each SMB Copper T 380A frame is made from compound of Low Density Polyethylene and Barium Sulphate (15% -24%) wound with 176mg of 0.25mm diameter copper wire, and two copper sleeves on horizontal arm providing a surface area of 380 mm2. The T is equipped with high-density polyethylene filament for easy removal. It is packed together with an Insertion Tube and Solid Rod in a Tyvek-Mylar film pouch ...
Manufactured by:SMB Corporation Of India based inMumbai, INDIA
Each SMB Cu 375 frame is made from compound of Low Density Polyethylene and Barium Sulphate (15% -24%) with two flexible arms with spurs. 99.99% purity OFE Copper wire (Diameter 0.40 mm ± 0.01 mm) is wound around the stem, giving a total copper surface area of 375mm2 ± 20mm2. The 'Frame' is equipped with Polyamide Monofilament(Suture)for checking the presence/position and easy ...
Manufactured by:SMB Corporation Of India based inMumbai, INDIA
Each SMB Cu 250 frame is made from compound of Low Density Polyethylene and Barium Sulphate(15% - 24%) with two flexible arms with spurs. 99-99% purity OFE Copper wire (Diameter 0.30 mm ± 0-01 mm) is wound around the stem, giving a total copper surface area of 250mm; ±20mm2. The 'Frame' is equipped with Polyamide Monofilament (Suture) for checking the presence/position of IUD and ...
Manufactured by:SMB Corporation Of India based inMumbai, INDIA
Each modified T shaped frame of SMB TCu 380 Plus is made from compound of Low Density Polyethylene and Barium Sulphate (15% -24%) wound with 0.40mm diameter copper wire providing a surface area of 380mm2 ± 23mm2. The T is equipped with polyamide monofilament thread (suture)for easy removal. It is packed together with an Insertion Tube and Rod in a Tyvek-Mylar film pouch or Mylar-Mylar film ...
Manufactured by:Yeasen Biotechnology (Shanghai) Co.,Ltd. based inShanghai, CHINA
Dextran sulfate sodium salt (DSS) is a polyanionic derivative of dextran produced by esterification of Dextran with chlorosulphonic acid. The sulfur content is approximately 17% which corresponds to an average of 1.9 sulfate groups per glucosyl residue of the dextran molecule. DSS has several characteristics: 1) polyanionic complex, soluble in water, forming a colorless aqueous solution; 2) high ...
Manufactured by:SMB Corporation Of India based inMumbai, INDIA
Each Y shaped SMB TCu 380Ag frame is made from compound of Low Density Polyethylene and Barium Sulphate (15% - 24%) wound with 0.40mm diameter copper wire with silver core of 0.1mm providing a surface area of 380mm2 ± 23mm2. The Y frame is equipped with Polyamide Monofilament thread (suture)for easy removal. It is packed together with an Insertion Tube and Rod in a Tyvek-Mylar film pouch ...
Manufactured by:RedHill Biopharma Ltd. based inTel-Aviv, ISRAEL
RHB-107 (INN: upamostat) (formerly MESUPRON) is a first-in-class, once-daily, orally-administered, potent inhibitor of serine proteases targeting multiple indications, including COVID-19, cancer, inflammatory lung diseases and gastrointestinal diseases. ...
Manufactured by:e-LinkCare Meditech Co.,Ltd. based inHangzhou, CHINA
UBREATH Exhalation analyzer (BA200) is a medical device designed & manufactured by e-LinkCare Meditech to associate with both FeNO and FeCO testing to provide rapid, precise, quantitative measurement in order to assist with clinical diagnosis and management such as asthma and other chronic airway ...
Manufactured by:JD Bioscience Inc. (JDB) based inBuk-gu, SOUTH KOREA
IBD, including ulcerative colitis (UC) and crohn’s disease (CD) can be caused by genetic and environmental factors and can induces uncontrolled activation of intestinal immune cells. Pro-inflammatory cytokines, such as IL-6 and TNF, produced by activated immune cells are suggested to drive the perpetuation of inflammation and tissue ...