- Home
- Equipment
- asia middle east
- introducer sheath
Refine by
Locations
- Asia & Middle East
- Afghanistan
- Armenia
- Azerbaijan
- Bahrain
- Bangladesh
- Bhutan
- Brunei Darussalam
- Cambodia
- China
- East Timor
- Georgia
- Guam
- India
- Indonesia
- Iran
- Iraq
- Israel
- Japan
- Jordan
- Kazakhstan
- Kuwait
- Kyrgyzstan
- Laos
- Lebanon
- Malaysia
- Maldives
- Mongolia
- Myanmar
- Nepal
- North Korea
- Oman
- Pakistan
- Palestinian Territories
- Philippines
- Qatar
- Russia
- Saudi Arabia
- Singapore
- South Korea
- Sri Lanka
- Syria
- Taiwan
- Tajikistan
- Thailand
- Turkmenistan
- United Arab Emirates
- Uzbekistan
- Vietnam
- Yemen
Introducer Sheath Equipment Supplied In Asia Middle East
10 equipment items found
Manufactured by:Kimal based inWorcester, UNITED KINGDOM
Reliable access when you need it. Achieve consistent access every time with our extensive range of introducer needles, sheaths and kits to support diagnostic procedures. Available in a comprehensive range of sizes and configurations, Kimal AngioGate® equips you with a reliable solution to support the access requirements of your ...
Manufactured by:NeuroSafe Medical Co., Ltd. based inZhuhai, CHINA
It achieves this by utilizing a self-expandable thrombus capturing retriever, paired with a flexible, tapered push wire and an introducer sheath for precise placement in microcatheters. The design also includes platinum alloy markers for fluoroscopic visibility, facilitating accurate guidance during procedures. This new generation of revascularization devices ...
Manufactured by:Cordis based inMiami Lakes, FLORIDA (USA)
Intended for percutaneous entry and guidance of catheters, the Cordis EMERALD Diagnostic Guidewire complements our diagnostic catheter and catheter sheath introducer lines. Performance, endurance and safety are built into each EMERALD Guidewire with solid tensile strength to minimize the likelihood of stretching or fracturing. ...
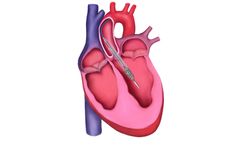
by:V-Wave Ltd. based inCaesarea Industrial Park (N), ISRAEL
The V-Wave Ventura Interatrial Shunt is a novel, hourglass-shaped implantable device. The shunt is designed to enable shunting of blood across the interatrial ...
Manufactured by:Cordis based inMiami Lakes, FLORIDA (USA)
The Cordis AVANTI+ Introducer is a pioneer of catheter sheath introducer technology. For use in arterial and venous procedures requiring percutaneous introduction of Intravascular devices. ...
Manufactured by:Cordis based inMiami Lakes, FLORIDA (USA)
The EXOSEAL VCD is indicated for femoral artery puncture site closure, reducing times to hemostasis and ambulation in patients who have undergone diagnostic or interventional catheterization procedures using a standard 5F, 6F, or 7F vascular sheath introducer with up to a 12-cm working ...
Manufactured by:Lifetech Scientific (Shenzhen) Co., Ltd. based inShenzhen, CHINA
The Aegisy™ Vena Cava Filter can be permanently placed in the IVC for PE prevention or can be removed out of the patient after a period of time (up to and including 14 days) as a retrievable filter. The Aegisy™ Vena Cava Filter is delivered with an Introducer Kit which contains a 6F Delivery Sheath, a Vessel Dilator, a 4.5F Delivery Cable and a 6F ...
Manufactured by:Lepu Medical Technology (Beijing) Co.,Ltd. based inChangping District, CHINA
High-performance balloon material. Puncture and abrasion-resistant. Controlled compliance for accurate balloon vessel sizing. Rigorous fatigue testing assures consistent ...
Manufactured by:Harland Medical Systems, Inc. based inEden Prairie, MINNESOTA (USA)
Harland’s Lubricent UV hydrophilic coatings provide exceptional friction reduction with state-of-the-art durability. Lubricent coatings enable catheters, sheaths, introducers, guide wires and other medical devices to navigate through the most difficult, tortuous vessels and anatomies. The solution blends a hydrogel-forming polymer with a proprietary ...
Manufactured by:Magenta Medical Ltd. based inKadima, ISRAEL
The Magenta percutaneous Left Ventricular Assist Device (pLVAD) is a self-expanding percutaneous catheter-mounted arterial pump that is intended to provide temporary mechanical circulatory support for the left ventricle (LV) during high-risk percutaneous coronary interventions (HR-PCI) and in patients hospitalized with cardiogenic shock (CS). In the course of HR-PCI and CS, the LV may require ...