- Home
- Equipment
- asia middle east
- microcatheter
Refine by
Locations
- Asia & Middle East
- Afghanistan
- Armenia
- Azerbaijan
- Bahrain
- Bangladesh
- Bhutan
- Brunei Darussalam
- Cambodia
- China
- East Timor
- Georgia
- Guam
- India
- Indonesia
- Iran
- Iraq
- Israel
- Japan
- Jordan
- Kazakhstan
- Kuwait
- Kyrgyzstan
- Laos
- Lebanon
- Malaysia
- Maldives
- Mongolia
- Myanmar
- Nepal
- North Korea
- Oman
- Pakistan
- Palestinian Territories
- Philippines
- Qatar
- Russia
- Saudi Arabia
- Singapore
- South Korea
- Sri Lanka
- Syria
- Taiwan
- Tajikistan
- Thailand
- Turkmenistan
- United Arab Emirates
- Uzbekistan
- Vietnam
- Yemen
Microcatheter Equipment Supplied In Asia Middle East
12 equipment items found
Manufactured by:OrbusNeich Medical Company Ltd. based inShatin, N.T., CHINA
Ultimate control and trackability for guidewire access in the most complex ...
Manufactured by:Medtronic based inMinneapolis, MINNESOTA (USA)
The Marathon™ flow directed microcatheter was designed for the infusion of therapeutic agents such as embolization materials and contrast media in tortuous, distal vessels.1 . The ApolloTM OnyxTM delivery microcatheter was expressly designed to access the neurovasculature for the controlled selective infusion of the OnyxTM liquid embolic system ...
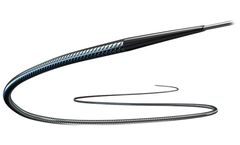
Manufactured by:Rapid Medical based inYokneam, ISRAEL
Net Length: 23 mm. Compatibility: 0.017” ID microcatheter. Diameter: 0.5 - 3 mm. Europ/Asia Cat. Number: TRPP3166. Canada Cat. Number: TRP5166. ...
Manufactured by:Rapid Medical based inYokneam, ISRAEL
Net Length: 20.5 mm. Compatibility: 0.016”/ 0.013” ID microcatheter. Diameter: 0.5 - 2.5 mm. Europ/Asia Cat. Number: TRPP3144. Canada Cat. Number: ...
Manufactured by:Rapid Medical based inYokneam, ISRAEL
Net Length: 53 mm. Compatibility: 0.021” ID microcatheter. Diameter: 1.5 - 9 mm. Europ/Asia Cat. Number: ...
Manufactured by:Rapid Medical based inYokneam, ISRAEL
Net Length: 22 mm. Compatibility: 0.017” ID microcatheter. Diameter: 0.5 - 3 mm. Europ/Asia Cat. Number: ANPP3199. Canada Cat. Number: ...
Manufactured by:Rapid Medical based inYokneam, ISRAEL
Net Length: 32 mm. Compatibility: 0.021” ID microcatheter. Diameter: 1.5 - 6 mm. Europ/Asia Cat. Number: TRPP3155. Canada Cat. Number: ...
Manufactured by:Rapid Medical based inYokneam, ISRAEL
Net Length: 24 mm. Compatibility: 0.021” ID microcatheter. Diameter: 1.5 - 3.5 mm. Europ/Asia Cat. Number: ANPP3188. Canada Cat. Number: ...
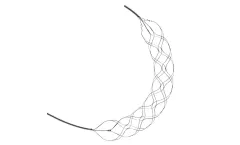
Manufactured by:Rapid Medical based inYokneam, ISRAEL
Comaneci: Net Length: 32 mm. Compatibility: 0.021” ID microcatheter. Diameter: 1.5 - 4.5 mm. Europ/Asia Cat. Number: ANPP3177. Canada Cat. Number: ...
Manufactured by:NeuroSafe Medical Co., Ltd. based inZhuhai, CHINA
It achieves this by utilizing a self-expandable thrombus capturing retriever, paired with a flexible, tapered push wire and an introducer sheath for precise placement in microcatheters. The design also includes platinum alloy markers for fluoroscopic visibility, facilitating accurate guidance during procedures. This new generation of revascularization devices overcomes ...
Manufactured by:Medtronic based inMinneapolis, MINNESOTA (USA)
Designed for presurgical embolization of brain arteriovenous malformations (bAVMs), Onyx™ liquid embolic system (LES) is a pre-mixed, radiopaque, injectable embolic fluid consisting of the following components: EVOH (ethylene vinyl-alcohol copolymer), DMSO (dimethyl-sulfoxide) and TA (micronized tantalum ...
Manufactured by:NeuroSafe Medical Co., Ltd. based inZhuhai, CHINA
The Renova Detachable Coil System is specifically designed for endovascular use to obstruct or occlude blood flow in vascular irregularities across neurovascular and peripheral vessels. Utilized for endovascular embolization, this system is effective in managing intracranial aneurysms, arteriovenous malformations, and arteriovenous fistulae, as well as performing arterial and venous embolizations ...