- Home
- Equipment
- australasia
- validates important clinical
Refine by
Validates Important Clinical Equipment Supplied In Australasia
11 equipment items found
Manufactured by:Integrated Endoscopy based inIrvine, CALIFORNIA (USA)
An Advanced, Single-use, Arthroscopic Innovation; Single-use nuvis is a surgical device that addresses the many issues hampering conventional reusable ...
Manufactured by:Avania based inBrunswick, AUSTRALIA
The right in vitro diagnostic (IVD) device can streamline clinical trials and expedite personalized patient care. We deliver world-class assistance for your IVD device development. We’ll help you build out effective research studies and protocols designed to validate algorithm reliability and ...
Manufactured by:Biotronik SE & Co. KG based inBerlin, GERMANY
Every year, more and more patients are receiving ICMs – all of which must be actively managed, creating additional workloads for everyone involved. BIOMONITOR III is the ICM that delivers easier and more efficient injection and monitoring, and our unique BIOvector design provides the clearest signals for easier evaluation and better informed ...
Manufactured by:SpeeDx based inSydney, AUSTRALIA
Single well qPCR test for detection of Neisseria gonorrhoeae and markers linked to ciprofloxacin susceptibility validated on a range of specimens and collection vessels. Antibiotic resistance in gonorrhoea infections is a global concern. There are limited effective treatment options and novel antibiotics are not readily available. Smarter and more directed use of antibiotics can be achieved ...
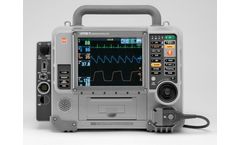
Manufactured by:Stryker Emergency Care based inKalamazoo, MICHIGAN (USA)
A monitor/defibrillator by professionals, for professionals. Rely on the LIFEPAK 15 monitor/defibrillator for the confidence you need in emergencies and the highest available escalating energy, up to 360 joules ...
Manufactured by:Proteomics International based inNedlands, Perth, AUSTRALIA
The ground-breaking prognostic test that can predict the onset of diabetic kidney disease and future kidney function decline in patients with type 2 diabetes. Diabetic kidney disease is the leading cause of chronic and end-stage kidney disease worldwide1. Most people with diabetic kidney disease do not have symptoms2. Prevention is better than cure - transform clinical ...
Manufactured by:Proteomics International based inNedlands, Perth, AUSTRALIA
Proteomics International has developed a versatile platform technology for discovering diagnostic tests based on the differences in the protein make-up of people with and without a particular disease. By comparing blood or other samples taken from both sick and healthy people, the Company is able to produce a set of “biomarkers”- biological signatures that can be used to test for a ...
Manufactured by:Care Essentials Pty Ltd. based inNorth Geelong, AUSTRALIA
The SARS-CoV-2 / COVID-19 Antigen Test Kit is an in vitro diagnostic rapid test used for the qualitative detection of the SARS-CoV-2 Antigen (Ag) in nasopharyngeal swab specimens from individuals with symptoms within a 7 day period. This test kit is for professional use only, by a health professional or a laboratory professional, and is intended to be used as an aid in the diagnosis of SARS-CoV-2 ...
Manufactured by:Orthocell Ltd. based inMurdoch, AUSTRALIA
Tendon injuries are one of the most common musculoskeletal complaints reported to general practitioners, with shoulder pain affecting more than 50% of adults over the age of 50. Mostly frequently, shoulder pain is the result of damage to the rotator cuff tendon. Rotator cuff tendon tears have a significant impact on the individual, not only affecting their ability to work or play sport, but also ...
by:Enigma based inAuckland, NEW ZEALAND
An advanced, web-based registry system to support data capture for acute coronary syndrome (ACS) management in secondary care after a patient has experienced an ACS event. The Registry collects standardised data from all ACS and Cathlab centres in New Zealand, allowing for benchmarking of services provided and detailed quality improvement to be ...
Manufactured by:ICU Medical, Inc. based inSan Clemente, CALIFORNIA (USA)
Industry-leading cybersecurity and complete IV-EHR interoperability, helping you reduce medication errors and increase patient safety. Is your IV smart pump smart enough? Despite nearly two decades of innovations that have helped improve patient safety—from dose-error reduction software (DERS) to IV-EHR interoperability—too many IV smart pumps on the market today still leave patient ...