Refine by
Disease Patient Equipment Supplied In Australia
9 equipment items found
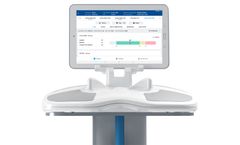
Manufactured by:ImpediMed Inc. based inCarlsbad, CALIFORNIA (USA)
SOZO uses ImpediMed’s BIS technology to measure and track critical information about the human body to aid clinicians in managing chronic disease and maximizing patient ...
Manufactured by:Mesoblast Ltd. based inMelbourne, AUSTRALIA
MPC-25-IC is Mesoblast’s Phase 2 product candidate for the treatment of acute myocardial infarction. This is the first clinical study to evaluate an allogeneic cellular therapy delivered by intracoronary infusion in patients who have suffered an acute myocardial ...
Manufactured by:Proteomics International based inNedlands, Perth, AUSTRALIA
The ground-breaking prognostic test that can predict the onset of diabetic kidney disease and future kidney function decline in patients with type 2 diabetes. Diabetic kidney disease is the leading cause of chronic and end-stage kidney disease worldwide1. Most people with diabetic kidney disease do not have ...
Manufactured by:Mesoblast Ltd. based inMelbourne, AUSTRALIA
MPC-25-Osteo for spinal fusion is a proprietary Phase 3-ready product candidate. All doses of MPC-25-Osteo for the treatment of spinal fusion consist of 25 million MPCs delivered on a collagen ceramic carrier material into the disc space with stabilizing ...
Manufactured by:Mesoblast Ltd. based inMelbourne, AUSTRALIA
Revascor is a Phase 3 product candidate being developed as a treatment for both advanced and end-stage chronic heart failure ...
by:AdAlta Limited based inBundoora, AUSTRALIA
AdAlta is utilising the power of the i-body technology platform to develop a growing pipeline of i-bodies to treat a range of conditions, with an initial focus on treating fibrotic diseases. AdAlta identified an i-body that binds to the drug target, CXCR4 and demonstrates anti-fibrotic effects in several models of fibrosis. This was initially called AD-114. Following further development, AdAlta ...
Manufactured by:Mesoblast Ltd. based inMelbourne, AUSTRALIA
MPC-06-ID is a Phase 3 product candidate for the treatment of chronic low back pain caused by disc degeneration (CLBP). It is being developed for patients who have exhausted conservative treatment options, may have failed epidural steroid injections and have no further treatment option other than invasive and costly surgical ...
Manufactured by:Mesoblast Ltd. based inMelbourne, AUSTRALIA
MPC-300-IV is being developed for the treatment of diabetic complications, including diabetic kidney disease known as diabetic ...
Manufactured by:TSI Incorporated based inShoreview, MINNESOTA (USA)
To limit the spread of disease through a facility, patients suspected of having diseases spread through the air must be placed in negative pressure rooms, airborne infection isolation rooms. Similarly, immune-compromised patients at risk of contracting diseases should stay in positive pressure, protective ...