- Home
- Equipment
- canada british columbia
- nasal swab
Refine by
Nasal Swab Equipment Supplied In Canada British Columbia
6 equipment items found
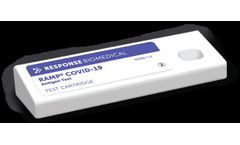
Manufactured by:Response Biomedical Corp. based inVancouver, BRITISH COLUMBIA (CANADA)
Our all-in-one RAMP® COVID-19 Antigen kit offers on the spot actionable results you can trust. Combined with our RAMP® 200 instrument, we offer rapid triage of nasal swab samples in 15 minutes. ...
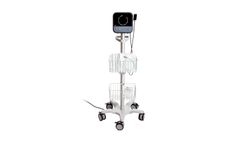
by:Ondine Biomedical Inc based inVancouver, BRITISH COLUMBIA (CANADA)
Reduce incidence of transmission and infections with photodisinfection. The nose is a primary home of bacteria, viruses, and fungi, which can be transmitted before any symptoms occur, adding risk to employees and their families. 1-4, 9. Handwashing, social distancing and masks alone are not sufficient to remove the risk of transmission. Photodisinfection is effective against infection-causing ...
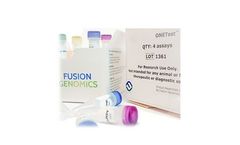
Manufactured by:Fusion Genomics Corporation based inBurnaby, BRITISH COLUMBIA (CANADA)
It contains all the reagents (including QUANTUMProbes) to produce an enriched sequenceable library from a variety of sample sources (blood, nasal wash, NP swab, etc.) within 6 ...
Manufactured by:Response Biomedical Corp. based inVancouver, BRITISH COLUMBIA (CANADA)
Response Biomedical provides a test that aids in the rapid diagnosis of RSV infections in symptomatic patients 21 years of age and younger. It detects RSV F-protein antigens in nasal wash, NP swab or NP aspirate specimens. It provides an objective and qualitative results in ~15 minutes. ...
Manufactured by:XPhyto Therapeutics Corporation based inVancouver, BRITISH COLUMBIA (CANADA)
XPhyto Therapeutics is focused on helping economies restart and businesses get up and running to bring life back to normal with a rapid, accurate and cost effective COVID ...
Manufactured by:Response Biomedical Corp. based inVancouver, BRITISH COLUMBIA (CANADA)
Response Biomedical provides a influenza test that aids in the rapid differential diagnosis of influenza viral infections in symptomatic patients. It identifies the presence of influenza A and influenza B nucleoprotein antigens in nasal wash/aspirate, NP swab or NP aspirate specimens. It provides an objective and qualitative results in ~15 minutes. ...