Refine by
Clinical Phase Equipment Supplied In Canada
11 equipment items found
Manufactured by:Hemostemix Inc. based inCalgary, ALBERTA (CANADA)
Hemostemix is currently conducting a randomized, double-blind, placebo-controlled Phase 2 clinical trial for patients with critical limb ischemia at sites in the Canada and United ...
Manufactured by:CanaQuest Medical Corp. based inMississauga, ONTARIO (CANADA)
Clinical testing on humans can only begin after a pre-clinical phase, involving laboratory studies (in vitro) and tests on animals, which has shown that the experimental drug is considered safe and effective. However, no animal is sufficiently similar to humans (even genetically modified ones) to make human testing unnecessary. ...
Manufactured by:Triple Hair Inc. based inDieppe, NEW BRUNSWICK (CANADA)
Its exclusive combination of molecules acts at various stages of the hair growth cycle to stop hair loss and stimulate regrowth, while also making hair thicker and darker. The effectiveness of this revolutionary solution was proven in the first two phases of clinical trials, where participants all observed positive results that ranged from moderate to ...
by:Zymeworks Inc based inVancouver, BRITISH COLUMBIA (CANADA)
ZW49 is a HER2-targeted antibody drug conjugate (ADC) developed using Zymeworks’ proprietary Azymetric™ and ZymeLink™ platforms. ZW49 is currently being evaluated in a Phase 1 clinical trial as a treatment for patients with locally advanced or metastatic HER2-expressing cancers that have progressed following treatment with existing approved ...
by:Enveric Biosciences, Inc. based inNaples, FLORIDA (USA)
Enveric Biosciences seeks to advance novel combinations of CBD with chemotherapeutic agents and immunotherapies. Preclinical data suggests combination therapies may improve the activity of certain chemotherapies or cancer immunotherapies, potentially enabling more potent or longer-lasting therapeutic effects. In addition, combination therapies may allow for the same or greater therapeutic effect ...
Manufactured by:CanaQuest Medical Corp. based inMississauga, ONTARIO (CANADA)
What are clinical trials? Clinical trials are a way to test new methods of diagnosing, treating, or preventing health conditions. The goal is to determine whether something is both safe and effective. A variety of things are evaluated through clinical trials, including: Effectiveness of medications, minimum and ultimate dose, medication ...
Manufactured by:Eupraxia Pharmaceuticals based inVictoria, BRITISH COLUMBIA (CANADA)
Eupraxia’s lead product candidate, EP-104IAR, is designed to meet the significant unmet medical need and market demand for long-lasting pain relief for knee osteoarthritis ...
Manufactured by:RepliCel Life Sciences based inVancouver, BRITISH COLUMBIA (CANADA)
RCS-01 is a proprietary autologous cell therapy being developed to rejuvenate aging or UV-damaged skin.* *This product is currently in clinical testing and not yet commercially available. RCS-01 is an autologous cell therapy utilizing non-bulbar dermal sheath (NBDS) cells - a type of fibroblast cell - isolated from the hair follicle to repair and regenerate tissue. Though several cell types are ...
Manufactured by:RepliCel Life Sciences based inVancouver, BRITISH COLUMBIA (CANADA)
RCT-01 is a proprietary autologous cell therapy being developed to treat chronically damaged tendons. * *This product is currently in clinical testing and not yet commercially available. RCT-01 is an autologous cell therapy utilizing non-bulbar dermal sheath (NBDS) cells - a type of fibroblast cell - isolated from the hair follicle to repair and regenerate tissue. Though several cell types are ...
Manufactured by:InMed Pharmaceuticals Inc. based inVancouver, BRITISH COLUMBIA (CANADA)
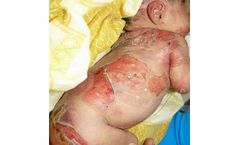
INM-755 is a cannabinol topical cream under development for the treatment of epidermolysis bullosa. INM-755 cream for EB is the first, and currently the only, cannabinol formulation being tested in clinical trials as a therapeutic ...
Manufactured by:Hemostemix Inc. based inCalgary, ALBERTA (CANADA)
Hemostemix’s technology uses a patient’s own cells to treat that patient’s disease. The cornerstone of this autologous technology is a novel cell population within the blood called the synergetic cell population (SCP). The synergetic cell population, which can be collected from a simple blood draw, consists of progenitor and other supporting cells that are being developed for ...