Show results for
Refine by
Clinical Regulations Equipment Supplied In Canada
5 equipment items found
Manufactured by:SeeMore Imaging Canada based inGibsons, BRITISH COLUMBIA (CANADA)
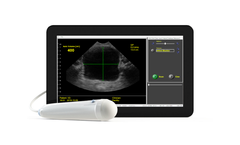
The ViewBladder10 tablet bladder scanner by SeeMore offers a comprehensive solution for non-invasive bladder volume measurement. Featuring multiple operational modes such as Quick Check Mode, the scanner allows fast determination of Post Void Residual Volume without the necessity of manual measurement, making a full bladder easily detectable. The Reference Mode integrates preloaded clinical ...
Manufactured by:Modus Medical Devices based inLondon, ONTARIO (CANADA)
The QUASAR Heavy Duty Respiratory Motion Platform is a programmable breathing and patient motion simulator for the quality assurance of motion-guided radiation therapy systems using any large-scale phantoms up to 45 kg (100 lbs). The Platform HD’s unique multi-directional motion simulation capability enables in-line translation with both the sagittal plane in the SI direction, or at a ...
Manufactured by:Modus Medical Devices based inLondon, ONTARIO (CANADA)
Radiation Therapy technologies, such as IGRT, SGRT and respiratory gating, have led to significant advances in patient treatment. The QUASAR™ Respiratory Motion Phantom (pRESP) is a programmable breathing and tumour motion simulator for end-to-end quality assurance on motion-guided radiation therapy systems including CT, LINAC, and PET. The QUASAR Respiratory Motion Phantom has been adopted ...
by:Theralase Technologies Inc. based inToronto, ONTARIO (CANADA)
Theralase Cool Laser Therapy ("CLT") medical devices are clinically indicated and cleared by the Food and Drug Administration (“FDA”) and Health Canada for the safe and effective treatment of knee pain. Theralase CLT devices are used off-label to eliminate pain, reduce inflammation and accelerate tissue healing to treat a broad range of nerve, muscle and joint conditions. ...
Manufactured by:Jubilant DraxImage Inc. dba Jubilant Radiopharma based inKirkland, Montréal, QUEBEC (CANADA)
Each kit consists of reaction vials which contain the sterile, non-pyrogenic, non-radioactive ingredients necessary to produce Technetium Tc 99m Albumin Aggregated Injection for diagnostic use by intravenous injection. Available in kits containing 30 reaction ...