Show results for
Refine by
Clinically Tested Equipment Supplied In Canada
16 equipment items found
Manufactured by:Shiphrah Biomedical Inc. based inToronto, ONTARIO (CANADA)
Introducing the PrenaBelt™, the first and only clinically-tested and published positional therapy device for use during sleep-in-pregnancy with internationally-demonstrated safety and efficacy in minimizing the amount of time spent sleeping on the back during late pregnancy in the home setting without otherwise disturbing sleep quantity or ...
Manufactured by:Valeo Pharma Inc. based inKirkland, QUEBEC (CANADA)
RedescaTM is the only heparin biosimilar that is clinically tested, has the largest global patient exposure, and that supprts affordability (health ...
Manufactured by:RepliCel Life Sciences based inVancouver, BRITISH COLUMBIA (CANADA)
RCH-01 is a proprietary autologous cell therapy being developed to treat pattern baldness.* *This product is currently in clinical testing and not yet commercially available. RCH-01 is an autologous cell therapy utilizing dermal sheath cup (DSC) cells isolated from the hair follicle to treat androgenetic alopecia. Though several cell types are involved, dermal ...
Manufactured by:CanaQuest Medical Corp. based inMississauga, ONTARIO (CANADA)
Clinical testing on humans can only begin after a pre-clinical phase, involving laboratory studies (in vitro) and tests on animals, which has shown that the experimental drug is considered safe and effective. However, no animal is sufficiently similar to humans (even genetically modified ones) to make human ...
Manufactured by:RepliCel Life Sciences based inVancouver, BRITISH COLUMBIA (CANADA)
RCS-01 is a proprietary autologous cell therapy being developed to rejuvenate aging or UV-damaged skin.* *This product is currently in clinical testing and not yet commercially available. RCS-01 is an autologous cell therapy utilizing non-bulbar dermal sheath (NBDS) cells - a type of fibroblast cell - isolated from the hair follicle to repair and regenerate ...
Manufactured by:RepliCel Life Sciences based inVancouver, BRITISH COLUMBIA (CANADA)
RCT-01 is a proprietary autologous cell therapy being developed to treat chronically damaged tendons. * *This product is currently in clinical testing and not yet commercially available. RCT-01 is an autologous cell therapy utilizing non-bulbar dermal sheath (NBDS) cells - a type of fibroblast cell - isolated from the hair follicle to repair and regenerate ...
Manufactured by:Advanced Electrophoresis Solutions Ltd. (AES) based inCambridge, ONTARIO (CANADA)
With our ISO:9001 certification, AES is proud to offer a comprehensive range of critical consumables, including proprietary cartridges, ampholytes, pI markers, electrolytes, and general solutions and consumables. In particular, we are pleased to supply high-quality 0.5% and 1% Methyl Cellulose (MC) solutions for icIEF analysis. Our MC solutions are fast, long-lasting, and compatible with ...
Manufactured by:Scion BioMedical based inMiami, QUEBEC (CANADA)
Our biopolymer-based Clo-Sur P.A.D. promotes hemostasis through its innovative formulation, structure and positive ionic charge. When Clo-Sur P.A.D. is applied to a wound, its positive charge immediately begins working to rapidly control bleeding. In-vitro laboratory studies have shown this occurs at a faster rate than many competing ...
Manufactured by:Citagenix Inc. based inLaval, QUEBEC (CANADA)
Neomem® FlexPlus is a single layer collagen membrane derived from porcine peritoneum. Suture pullout testing has shown this membrane to be biomechanically strong yet conformability evaluations demonstrate the ability to adapt to the contour of the surgical site. Neomem® FlexPlus hydrates quickly and handling characteristics make surgical placement easy. It can be repositioned easily and ...
Manufactured by:Pigg-O-Stat based inClifton, TENNESSEE (USA)
The World’s Top Clinically-Proven, Time-Tested, and Medically-Trusted Pediatric Foot Immobilizer. With five decades of success with the original analog Pigg-O-Stat™ Pediatric Immobilizer, a significant need remained. There are common childhood orthopedic cases every year where young kids experience flatfeet, toe walking, pigeon toes, and of course ...
Distributed by:Green Cross Medico Ltd based inHamilton, ONTARIO (CANADA)
Airglove is a worldwide patented air warming system developed to enable venipuncture and difficult intravenous access in a patients arm for the delivery of intravenous drugs. It gently heats the patients arm up as it forces warm air through a double walled polythene glove. ...
Manufactured by:Augurex Life Sciences Corp. based inVancouver, BRITISH COLUMBIA (CANADA)
Extracellular 14-3-3η protein is a potent ligand and activator of intracellular signalling pathways that lead to the up-regulation of inflammation and joint damage factors involved in RA pathogenesis. The 14-3-3η blood test is clinically available as a diagnostic test whereby a positive result indicates a 5 to 50 times greater likelihood ...
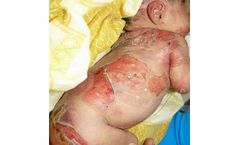
Manufactured by:InMed Pharmaceuticals Inc. based inVancouver, BRITISH COLUMBIA (CANADA)
INM-755 is a cannabinol topical cream under development for the treatment of epidermolysis bullosa. INM-755 cream for EB is the first, and currently the only, cannabinol formulation being tested in clinical trials as a therapeutic ...
Manufactured by:Angany Inc. based inSt-Jean, QUEBEC (CANADA)
We aim to provide safe, dependable and lasting relief from allergy. We have developed a unique therapeutic approach based on a proprietary allergy vaccine technology to address the specific challenge of allergy (eBioparticle-Potentiated ...
Manufactured by:BioMark Diagnostics Inc. based inRichmond, BRITISH COLUMBIA (CANADA)
BioMark’s initial liquid biopsy assay detects SSAT1 using an FDA approved drug. SSAT1 is an enzyme with elevated levels in numerous cancers. The assay targets a well researched and understood metabolic pathway. BioMark of a few unique companies using safe therapeutic agents for diagnostic indication. As a non-invasive cost effective red alert tool, it is valuable to physicians around the ...
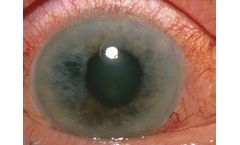
Manufactured by:InMed Pharmaceuticals Inc. based inVancouver, BRITISH COLUMBIA (CANADA)
INM-088 is a topical eye drop formulation under development for the treatment of glaucoma. The active pharmaceutical ingredient (API) in INM-088 is cannabinol, also known as CBN, a rare cannabinoid showing promise in its potential to provide neuroprotection and to reduce intraocular pressure of the ...