Refine by
Applications
- Better Tool for Physicians
- Portable Handheld Ultrasound Technology for Fatty Liver Disease Patients
- Portable Handheld Ultrasound Technology for Viral & Autoimmune Liver Disease Patients
- Portable Handheld Ultrasound Technology for Clinical Trials
- Technology for Surface Modification and Drug Delivery
- Medical cannabis solutions for regulatory pathway sector
- Medical cannabis solutions for mental health costs sector
In Vitro Equipment Supplied In Canada
24 equipment items found
Manufactured by:Agilent Technologies, Inc. based inSanta Clara, CALIFORNIA (USA)
The Cas9 endonuclease kit is part of an integrated solution for in-vitro CRISPR/Cas resesarch. Use this kit for in-vitro cloning of large genes or DNA fragments without the limitations imposed by common restriction enzymes or PCR fidelity. Allows cleavage of DNA at single and multiple locations based on a custom sequence of interest. Customizable endonuclease ...
Manufactured by:Scion BioMedical based inMiami, QUEBEC (CANADA)
When Clo-Sur P.A.D. is applied to a wound, its positive charge immediately begins working to rapidly control bleeding. In-vitro laboratory studies have shown this occurs at a faster rate than many competing ...
Manufactured by:GenomeMe Lab Inc. based inRichmond, BRITISH COLUMBIA (CANADA)
The GeneNav™ HPV One qPCR Kit is designed for quick initial screening of individuals for the presence of all 14 High Risk human papilloma virus (HPV) subtypes allowing physicians to identify those at risk for cervical cancer. This in vitro diagnostic kit allows for the specific detection and discrimination between HPV 16, HPV 18, and nonspecific pooled detection of the ...
Manufactured by:Citagenix Inc. based inLaval, QUEBEC (CANADA)
Neomem® FlexPlus hydrates quickly and handling characteristics make surgical placement easy. It can be repositioned easily and placed wet or dry and with either side up. Additionally, in vitro testing of Neomem® FlexPlus shows low tissue inflammation and foreign body giant cell ...
Manufactured by:Canadian Hospital Specialties Limited (CHS) based inOakville, ONTARIO (CANADA)
Their goal is to develop more effective, natural and economically viable treatments for Chronic Wounds. The first of its kind technology enables health care providers to produce in real time – an in vitro blood clot from a patient’s whole blood. Once applied the blood clot serves as a protective covering and supports wound healing processes which naturally occur in ...
Manufactured by:Agilent Technologies, Inc. based inSanta Clara, CALIFORNIA (USA)
The SureGuide gRNA Synthesis Kit is part of an integrated solution for in-vitro CRISPR/Cas research. Use this kit to generate high concentrations of gRNA, and combine with the SureGuide Cas9, or your own Cas9 protein, to clone complex genomic regions with high accuracy and specificity. The SureGuide gRNA kit shortens your time from planning to experiment, and reduces the cost of ...
Manufactured by:BTNX Inc. based inPickering, ONTARIO (CANADA)
This oral fluid test is a rapid, qualitative screening test that simultaneously detects Marijuana (THC), at the cut-off level of 30 ng/mL, and/or Cotinine (COT), at the cut-off level of 40 ng/mL, in human oral fluid. This multi panel drug test cup can provide accurate and fast results in 10 minutes. It is intended for in vitro diagnostic use ...
Manufactured by:StressMarq Biosciences Inc. based inVictoria, BRITISH COLUMBIA (CANADA)
Conjugates: ATTO 390, ATTO 488, ATTO 594, Biotin, FITC, Unconjugated. Field Of Use: Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use ...
Manufactured by:StressMarq Biosciences Inc. based inVictoria, BRITISH COLUMBIA (CANADA)
Conjugates: ATTO 390, ATTO 488, ATTO 594, Biotin, FITC, Unconjugated. Field Of Use: Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use ...
Manufactured by:GenomeMe Lab Inc. based inRichmond, BRITISH COLUMBIA (CANADA)
In addition, HPV Complete enables detection of HPV types 6 and 11 which are considered low risk for cervical cancer, however they are the cause of 90% of all cases of genital warts as well as respiratory papillomatosis. This in vitro diagnostic kit allows for simultaneous detection of HPV 16 or HPV 18, nonspecific pooled detection of the other 12 high risk HPV subtypes (HPV 31, ...
Manufactured by:eNUVIO inc. based inMontreal, QUEBEC (CANADA)
Get even more out of your neuronal cultures and co-cultures with the latest in microfluidic devices from eNUVIO. OMEGAACE devices can be used for in vitro triple co-culture studies of neurodegeneration, neuromuscular junction (NMJ), nigrostriatal pathway, pain, myelination, or chemotactic factors. In single culture applications, the OMEGAACE can also serve as a ...
Manufactured by:eNUVIO inc. based inMontreal, QUEBEC (CANADA)
Get more out of your neuronal cultures and co-cultures with the latest in microfluidic devices from eNUVIO. OMEGA4 devices can be used for in vitro co-culture studies of neurodegeneration, neuromuscular junction (NMJ), nigrostriatal pathway, pain, myelination, or chemotactic factors. In single culture applications, the OMEGA4 can serve as a compartmentalization tool to separate ...
Manufactured by:eNUVIO inc. based inMontreal, QUEBEC (CANADA)
Each experimental unit consists of one standard sized chamber and one small chamber. OMEGA4-2mini devices can be used for in vitro co-culture studies of neurodegeneration, neuromuscular junction (NMJ), nigrostriatal pathway, pain, myelination, or chemotactic factors. In single culture applications, the OMEGA4-2mini can serve as a compartmentalization tool to separate the somatic ...
Manufactured by:eNUVIO inc. based inMontreal, QUEBEC (CANADA)
Each experimental unit consists of one standard sized chamber and one small chamber. OMEGA4-2mini-2mm devices can be used for in vitro co-culture studies of neurodegeneration, neuromuscular junction (NMJ), nigrostriatal pathway, pain, myelination, or chemotactic factors. In single culture applications, the OMEGA4-2mini-2mm can serve as a compartmentalization tool to separate the ...
Manufactured by:BioMedica Diagnostics based inWindsor, NOVA SCOTIA (CANADA)
ACTICHROME Heparin (Anti-FIIa) is an in vitro diagnostic three-stage assay. It begins with plasmas containing heparin interacting with human antithrombin III to form a heparin-ATIII complex. In the second stage, thrombin is added, which the complex inhibits. The third stage involves the residual thrombin activity hydrolyzing SPECTROZYME TH, a chromogenic substrate. ...
Manufactured by:CanaQuest Medical Corp. based inMississauga, ONTARIO (CANADA)
Clinical testing on humans can only begin after a pre-clinical phase, involving laboratory studies (in vitro) and tests on animals, which has shown that the experimental drug is considered safe and effective. However, no animal is sufficiently similar to humans (even genetically modified ones) to make human testing unnecessary. For this reason, the experimental drug must also be ...
Manufactured by:StressMarq Biosciences Inc. based inVictoria, BRITISH COLUMBIA (CANADA)
Conjugates: ATTO 390, ATTO 488, ATTO 594, Biotin, FITC, Unconjugated. Field Of Use: Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use ...
Manufactured by:StressMarq Biosciences Inc. based inVictoria, BRITISH COLUMBIA (CANADA)
Conjugates: ATTO 390, ATTO 488, ATTO 594, Biotin, FITC, Unconjugated. Field Of Use: Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use ...
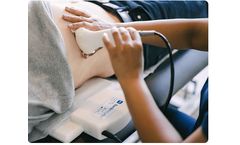
Manufactured by:Sonic Incytes Medical Corp. based inVancouver, BRITISH COLUMBIA (CANADA)
Velacur is a portable, handheld ultrasound that integrates a live ultrasound image, resulting in an assessment of liver stiffness and attenuation, components of liver fibrosis and fat measurements. It generates real-time stiffness measurements at point of care with technology similar to that of MRI elastography. Velacur is safe and effective as an aid in the management of patients with liver ...
Manufactured by:Covalon Technologies Ltd based inMississauga, ONTARIO (CANADA)
ColActive Transfer is pliable, absorbent, and biocompatible, and is designed to intimately contact the wound bed and protect it from secondary dressings (i.e. synthetic foam). ColActive® Transfer has low adherence, which helps minimize wound trauma at dressing ...