Show results for
Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
Biological Safety Equipment Supplied In Europe
24 equipment items found
Manufactured by:Anthogyr - Institut Straumann AG based inSallanches, FRANCE
Axiom® Tissue Level implant promotes biological safety and easy prosthesis insertion at the gingival level. It helps to preserve the epithelium and connective tissue attachment. The Axiom® TL implant is available in the REG profile, suitable for most clinical indications, and PX profile preferred for immediate post-extraction implantation for better ...
Manufactured by:OriGene Technologies, Inc. based inRockville, MARYLAND (USA)
OriGene offers pre-made lentiviral shRNA plasmid kits containing 4 sequence-verified expression cassettes that target your gene of interest. Each lentiviral cassette contains three major functional elements: shRNA, a puromycin selection marker & a tGFP reporter. With a guaranteed knockdown of ≥70%, these kits and our ready-to-use lenti-shRNA particles are a great option for studying ...
Manufactured by:MR Solutions based inGuildford, UNITED KINGDOM
The MRS*DRYMAG 9.4T is a cutting-edge, cryogen-free MRI scanner designed for preclinical imaging of animals up to 3 kg. Utilizing proprietary Dry Magnet technology, this system does not require liquid helium or nitrogen for cooling, making installation achievable even in biosafety labs with minimal site preparation. Its compact design weighs merely 700 kg compared to conventional wet magnets ...
by:PatoGen Analyse AS based inAalesund, NORWAY
PatoGen BioSafety Program™ is a comprehensive system for minimising the risk of disease and loss throughout the production cycle. PatoGen's aim is for the program both to prevent the introduction of infection as a result of a lack of knowledge of infection status in individual populations and enable potential disease problems to be ...
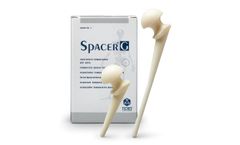
Manufactured by:Tecres S.p.A. based inSommacampagna (VR), ITALY
Spacer®-G is the preformed spacer for hip preloaded with Gentamicin. The device includes a load-bearing structure in stainless steel, with a standard tapered stem. It’s available in 3 different head sizes (46-54-60mm) and 2 different stem ...
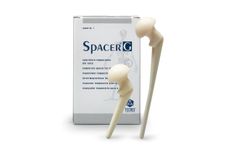
Manufactured by:Tecres S.p.A. based inSommacampagna (VR), ITALY
Spacer®-G Flat Stem is the preformed spacer for hip preloaded with Gentamicin. The device includes a load-bearing structure in stainless steel, with a rectangular flat stem. It’s available in 3 different head sizes (46-54-60mm) and 2 different stem ...
Manufactured by:Aseptic Technologies based inGembloux, BELGIUM
The Crystal M1 Filling Station is a tabletop system designed for aseptic filling processes, providing a reliable solution for manual fill and finish operations. It integrates AT-Closed Vial® technology, which keeps the vial functionally closed during the entire filling process, thereby reducing contamination risks. This system consists of a filling unit, a dosing system, precision balance, ...
Manufactured by:Regenhu based inVillaz-St-Pierre, SWITZERLAND
The 3d bioprinting station in a biosafety enclosure: bringing the future of tissue engineering and regenerative medicine closer by revolutionizing medicine ...
Manufactured by:ViruSure GmbH based inVienna, AUSTRIA
Gene therapy has brought hope to many diseases with unmet clinical need. The potential to permanently cure the patient through replacement of a defective gene with a healthy gene underscores the surge of interest in this exciting biopharma ...
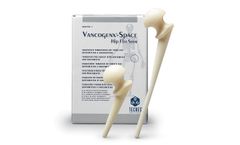
Manufactured by:Tecres S.p.A. based inSommacampagna (VR), ITALY
Vancogenx®-Space Hip is the preformed spacer for hip preloaded with Gentamicin and Vancomycin. The device includes a load-bearing structure in stainless steel, with a standard tapered stem. It’s available in 3 different head sizes (46-54-60mm) and 2 different stem ...
Manufactured by:Anthogyr - Institut Straumann AG based inSallanches, FRANCE
Axiom® Multi Level® is an implant concept that offers a smart range of surgical solutions and complementary philosophies between Bone and Tissue Level implants. Axiom® Multi Level® philosophy is fully driven by prosthetics outcome for better patients satisfaction, with a strong orientation for digital and CAD/CAM custom-made restorations. ...
Manufactured by:Genenta Science based inMilano, ITALY
Temferon™ is a lenti-virus based hematopoietic stem progenitor cell immuno-gene therapy enabling controlled and targeted interferon-α expression within cancers. Biologically delivered via engineered tumor infiltrating monocytes (Tie2 expressing monocytes – TEMs), Temferon™ generates immune responses to any TEMs positive tumors. As such the ...
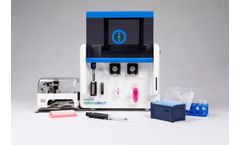
Manufactured by:NanoCellect Biomedical, Inc. based inSan Diego, CALIFORNIA (USA)
A module for the WOLF Cell Sorter to sort and dispense into 96-well or 384-well plates. Applications requiring the isolation, sorting and dispensing of single cells, such as B-cell identification for antibody discovery, or monoclonal CHO cell line development, are now easier with the N1 Single-Cell Dispenser. Additionally, single-cell genomics assays can be performed where single cells are plated ...
Manufactured by:Mediso Ltd. based inBudapest, HUNGARY
The MultiScan™ Large Field of view Extreme Resolution PET/CT system is offering molecular imaging with high spatial resolution and high quantitative accuracy in a gantry that supports all possible non-human primate (NHP) studies. Fine crystal needles and advanced detector modelling of the Tera-Tomo™ 3D PET reconstruction enable to resolve 1 mm structures. Our highly refined detector ...
Manufactured by:OraSure Technologies based inBethlehem, PENNSYLVANIA (USA)
The OraQuick® Ebola Rapid Antigen Test has been granted De Novo 510(k) clearance by the U.S. Department of Health and Human Services for the following intended use: The OraQuick® Ebola Rapid Antigen Test is an in vitro diagnostic single-use immunoassay for the qualitative detection of antigens from viruses within the Ebolavirus genus but does not differentiate between these viruses. ...
Manufactured by:Noraker based inLyon, FRANCE
Functional and aesthetic restoration are daily challenges for dental surgeons. The choice of treatment and implants and high performance bone substitutes are the answer to the specificity of clinical cases. ...
Manufactured by:Noraker based inLyon, FRANCE
Functional and aesthetic restoration are daily challenges for dental surgeons. The choice of treatment and implants and high performance bone substitutes are the answer to the specificity of clinical ...
Manufactured by:OraSure Technologies based inBethlehem, PENNSYLVANIA (USA)
The OraQuick® Ebola Rapid Antigen Test has been granted De Novo 510(k) clearance by the U.S. Department of Health and Human Services for the following intended use: The OraQuick® Ebola Rapid Antigen Test is an in vitro diagnostic single-use immunoassay for the qualitative detection of antigens from viruses within the Ebolavirus genus but does not differentiate between these viruses. ...
Manufactured by:NanoCellect Biomedical, Inc. based inSan Diego, CALIFORNIA (USA)
The WOLF was originally created by a team of scientists and engineers who wanted to solve a classic challenge in biological research: how to sort cells effectively, easily, and of high quality. Now, as science continues to move forward into increasingly complex realms, the WOLF is moving forward with it. The new WOLF G2 instrument has significantly expanded the capabilities of gentle benchtop ...
Distributed by:Vacuum Service based inRoma, ITALY
Solviva is a family of biomaterials offered for use in implantable medical devices. Only products designated as part of the Solviva family of biomaterials may be considered as candidates for medical applications implanted in the human body and devices that are in contact with bodily fluids or tissues for greater than 24 ...