Show results for
Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
Bone Marrow Mononuclear Cells Equipment Supplied In Europe
13 equipment items found
Manufactured by:Precision For Medicine, Inc. based inFrederick, MARYLAND (USA)
Advancing your research into hematological malignancies, immunology, and other related areas requires access to high quality human bone marrow mononuclear cells. Precision for Medicine has an extensive inventory of normal, healthy donors with longitudinal data and ability to recall for additional biospecimen ...
Manufactured by:DSM based inHeerlen, NETHERLANDS
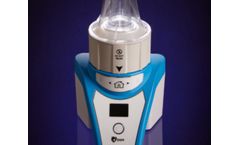
Bring more consistent outcomes to your portfolio with LAVA™ cell concentration technology, offering high cell yield, the shortest processing time and the smallest and lightest centrifuge design. The LAVA™ family of devices separates and concentrates biologic fluids to create supraphysiologic cell solutions such as Platelet Rich Plasma and Bone Marrow Concentrates. LAVA™ cell ...
Manufactured by:Novadip Biosciences based inMont-Saint-Guibert, BELGIUM
Novadip’s unique “3M3” platform for tissue regeneration combines over 15 years of academic research with practical surgery experience and proof of principle clinical data. ...
by:Gain Therapeutics, Inc. based inBethesda, MARYLAND (USA)
Morquio B, also known as Mucopolysaccharidosis type IV (MPS IV), is a progressive disease mostly impacting the skeleton, caused by mutations in GLB1, the gene that encodes the beta-galactosidase (GLB) enzyme. These mutations result in the misfolding and subsequent dysfunction of GLB, which leads to the toxic substrate accumulation of keratan sulfate in organs and tissues. Very limited and ...
Manufactured by:CTIBiotech based inMeyzieu, FRANCE
CTIBIOTECH is one of the few SME companies in Europe licensed as a human tissue/cell biobank. The CTIBIOBANK stores cells and tissues which are used internally and with our ...
Manufactured by:MDL Srl based inDelebio, ITALY
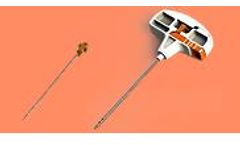
Selective multiradial, micro-aspiration system of autologous bone marrow tissue for stem cell therapy. ...
Manufactured by:ADS Biotec Inc. based inOmaha, NEBRASKA (USA)
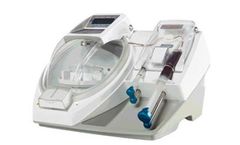
The HANABI-PII Plus reduces hands-on time of the entire harvesting process by 75%, allowing time for other specialized tasks and providing increased throughput for your laboratory. Capable of processing up to 24 samples per run and it eliminates variability compared to manual processing methods. The HANABI-PII Plus can be integrated with additional options such as reagent sensors, waste fluid ...
Manufactured by:ADS Biotec Inc. based inOmaha, NEBRASKA (USA)
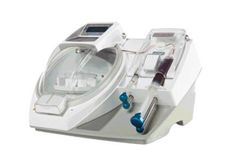
The HANABI-PI is specifically created for small to mid-size throughput, enabling processing of up to 16 suspension cultures per run and eliminating variability compared to manual processing methods. Walk away reliability and low maintenance reduces FTE time associated with the manual harvesting process up to 75%. The HANABI-PI can be integrated with additional options such as reagent sensors, ...
by:Gain Therapeutics, Inc. based inBethesda, MARYLAND (USA)
GM1 Gangliosidosis is a hereditary, progressive disease mostly impacting neurons in the brain and spinal cord, caused by mutations in GLB1, the gene that encodes the beta-galactosidase (GLB) enzyme. These mutations result in the misfolding and subsequent dysfunction of GLB, which leads to the toxic substrate accumulation of GM1 ganglioside in organs and tissues. Very limited and investigational ...
Manufactured by:ADS Biotec Inc. based inOmaha, NEBRASKA (USA)
The HANABI-PIII Plus reduces hands-on time of the entire harvesting process by 75%, allowing time for other specialized tasks, providing increased throughput for your laboratory. Specifically created for large-size samples throughput, the HANABI-PIII Plus enables processing up to 64 samples per run and eliminates variability compared to manual processing methods. The HANABI-PIII Plus can be ...
Manufactured by:Global Biyomedikal based inHalkali Küçükçekmece Istanbul, TURKEY
Orthopedics and Physical Treatment PRP Applications for Only One-Session 25-Times denser concentration can be achieved from a Standard PRP System through Magellan System. Magellan Autologous Platelet Separator System is a biological concentration and transmission system that separates and concentrates the platelet within patient’s own blood. ...
Manufactured by:Global Biyomedikal based inHalkali Küçükçekmece Istanbul, TURKEY
Orthopedics and Physical Treatment PRP Applications for Only One-Session 25-Times denser concentration can be achieved from a Standard PRP System through Magellan System. Magellan® Autologous Platelet Separator System is a biological concentration and transmission system that separates and concentrates the platelet within patient’s own ...
by:Aurealis Therapeutics based inBasel, SWITZERLAND
Our new approach to chronic wound healing moves far beyond symptom ...