Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
Cardiogenic Shock Equipment Supplied In Europe
5 equipment items found
Manufactured by:Abiomed based inDanvers, MASSACHUSETTS (USA)
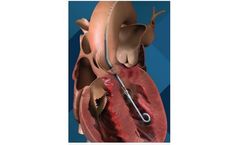
The Impella 2.5 heart pump temporarily maintains blood flow during high-risk PCI procedures or cardiogenic shock. It allows the heart to rest and pumps blood to vital organs while the physician treats the underlining ...
Manufactured by:Abiomed based inDanvers, MASSACHUSETTS (USA)
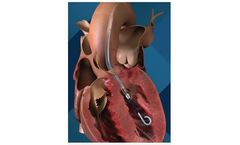
The Impella CP with SmartAssist heart pump is designated as safe and effective by the FDA for use during high-risk PCI procedures and for patients in cardiogenic shock. It is a minimally invasive, temporary heart pump that uses real-time intelligence associated with improved survival and heart ...
Manufactured by:Abiomed based inDanvers, MASSACHUSETTS (USA)
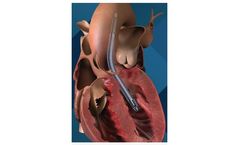
The Impella 5.5 with SmartAssist heart pump delivers full cardiac support, allowing the heart to rest and enabling the heart to achieve its natural pumping function without additional support. This heart pump is designed for long-duration support, enables patient mobility and optimizes recovery by using real-time ...
Manufactured by:Abiomed based inDanvers, MASSACHUSETTS (USA)
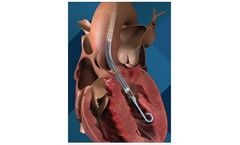
Impella 5.0 is a temporary, minimally invasive heart pump that provides circulatory support, enabling the heart to rest and recover. Impella LD is a surgically implanted heart pump that provides temporary support to the heart during and after a procedure. Impella 5.0 and Impella LD heart pumps allow patients to walk around while on support to improve recovery, if inserted via the axillary artery ...
Manufactured by:CytoSorbents Corporation based inMonmouth Junction, NEW JERSEY (USA)
he CytoSorb therapy* is based on an extracorporeal blood purification procedure that was shown to effectively reduce excessive levels of inflammatory mediators (see “The Adsorber”). This is intended to alleviate the excess systemic inflammatory response (“cytokine storm”) associated with systemic hyperinflammation or septic shock. Thus, the life-threatening complications ...